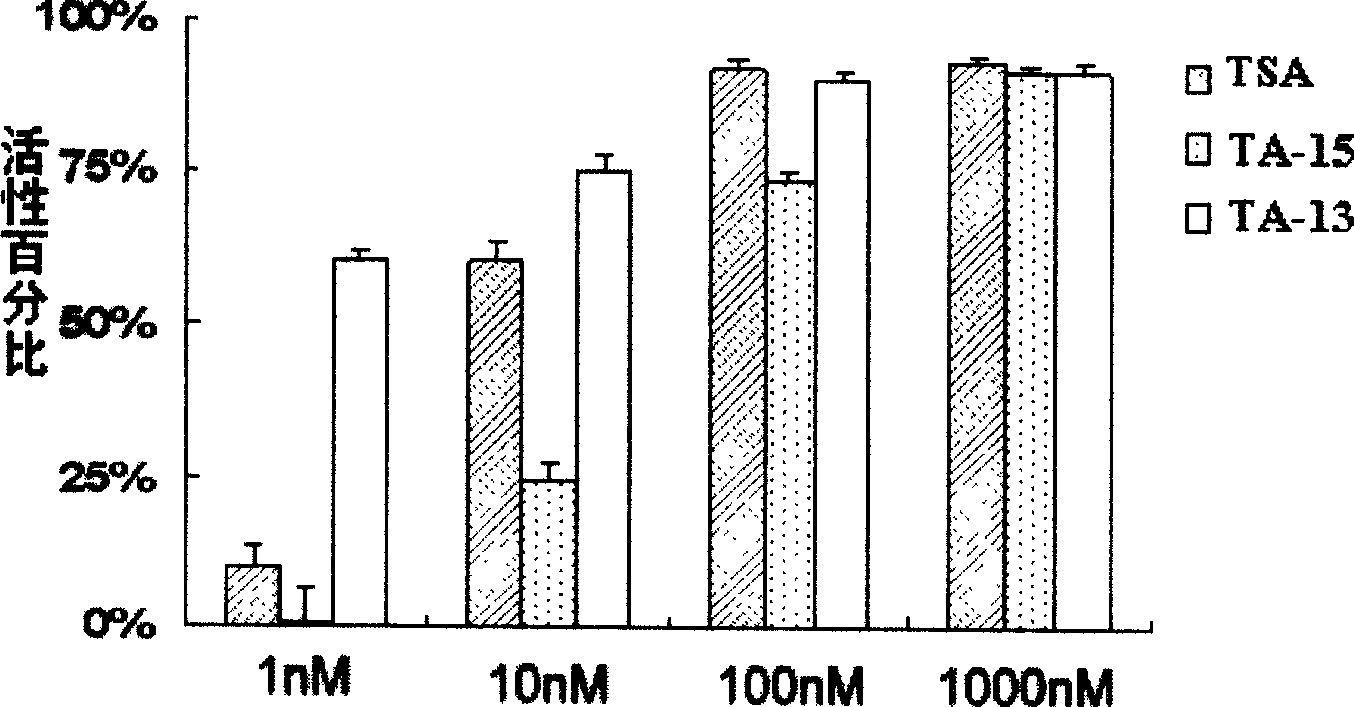
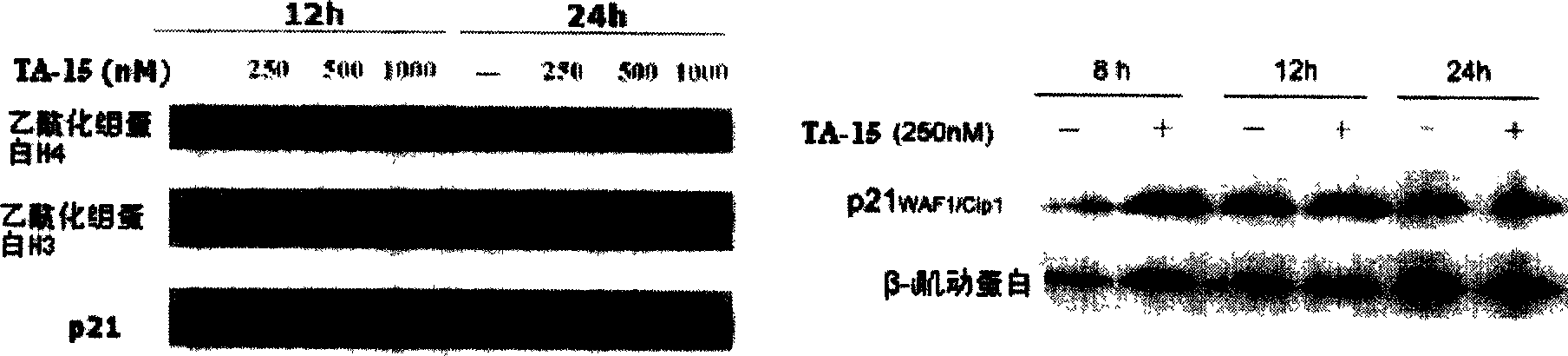
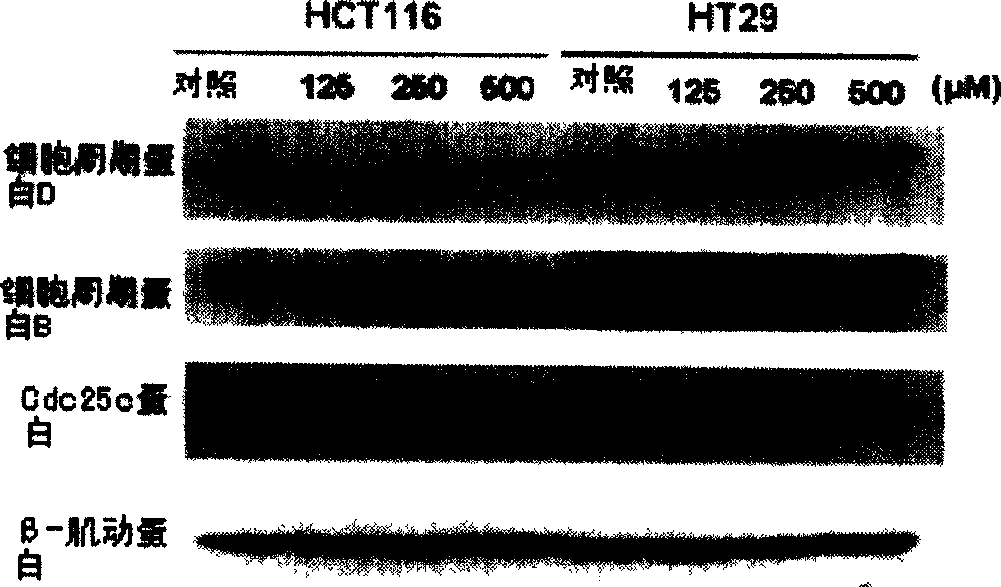
Trichostatin A derivatives, preparation method and use thereof
A technology for trichostatin and derivatives, applied in the field of trichostatin A derivatives and their preparation, can solve the problems of loss of clinical application, difficulty in synthesis, unstable metabolism and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: (R, 2E, 4E)-7-(4-aminophenyl)-N-hydroxyl-4,6-dimethyl-7-oxo-2,4-dieneheptamide (TA- 1) Preparation
[0060] A: Preparation of intermediate (2E, 4E 6R, 7R)-7-(4-aminophenyl)-7-hydroxyl-4,6-dimethyl-2,4-dieneheptanoic acid ethyl ester (its preparation Refer to the patent application: application number 200510030149.5)
[0061] (1) Dissolve 1.51g of p-nitrobenzaldehyde in 35ml of DMF, add 230mg of L-proline, then add 1.44ml of propionaldehyde, and stir at room temperature for 7 hours. Add 100ml of water, extract three times with ethyl acetate, combine the organic layers, dry over magnesium sulfate, filter, and concentrate the filtrate to obtain the crude product, which is directly put into the next step.
[0062] (2) Dissolve the crude product obtained in the previous step in 30ml methylene chloride, add 9.6g wittig reagent Ph 3 P=C(CH 3 )CO 2 Me, heated to reflux for 12 hours, evaporated to dryness, silica gel column chromatography (ethyl acetate / petroleum...
Embodiment 2
[0074] Example 2: (R, 2E, 4E)-7-(4-(diethylamino)phenyl)-N-hydroxyl-4,6-dimethyl-7-oxo-2,4-dienehepta Preparation of amides (TA-2)
[0075] A: 150 mg of the intermediate (2E, 4E 6R, 7R)-7-(4-aminophenyl)-7-hydroxyl-4,6-dimethyl-2,4- Dissolve ethyl diene heptanoate in 5ml tetrahydrofuran, add 685mg of 40% acetaldehyde aqueous solution (3eq.), stir at room temperature for 10 minutes, add 530mg sodium triacetoxyborohydride, continue stirring at room temperature for 10 hours, add saturated NaHCO 3solution, was extracted with ethyl acetate, and the organic phases were combined. The organic phase was washed with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated. The residue was purified by silica gel column chromatography (ethyl acetate / petroleum ether=1 / 5 (V / V)) to obtain 148 mg of (2E, 4E, 6R, 7R)-(4-(diethylamino)phenyl)- Ethyl 7-hydroxy-4,6-dimethyl-2,4-dieneheptanoate (yield: 83%).
[0076] B: The product obtained in the pr...
Embodiment 3
[0079] Example 3: (R, 2E, 4E)-7-(4-(dipropylamino)phenyl)-N-hydroxy-4,6-dimethyl-7-oxo-2,4-dienehepta Preparation of Amide (TA-3)
[0080] The operation was the same as in Example 2, substituting propionaldehyde for acetaldehyde to obtain the title compound with the following structure.
[0081] 1 H-NMR (CD 3 OD: CDCl 3 =5:1, 300Hz, δppm): d=7.68(2H, d, J=9.3Hz), 7.07(1H, d, J=15.6Hz), 6.49(2H, d, J=9.3Hz), 5.82( 1H, d, J = 10.2Hz), 5.66 (1H, d, J = 15.6Hz), 4.26 (1H, m), 3.22 (4H, t, J = 7.2Hz), 1.77 (3H, s), 1.50 ( 4H, m), 1.17 (3H, d, J=6.6Hz), 0.82 (6H, t, J=7.2Hz)
[0082]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com