Novel method for preparing indissoluble medicaments liposome preparations
A technology of liposomes and blank liposomes, which is applied in liposome delivery, pharmaceutical formulations, drug combinations, etc., can solve the problems of unguaranteed stability of preparations, inconvenient clinical operations, adverse reactions, etc., and achieve good dilution stability , Avoid toxic and side effects, improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
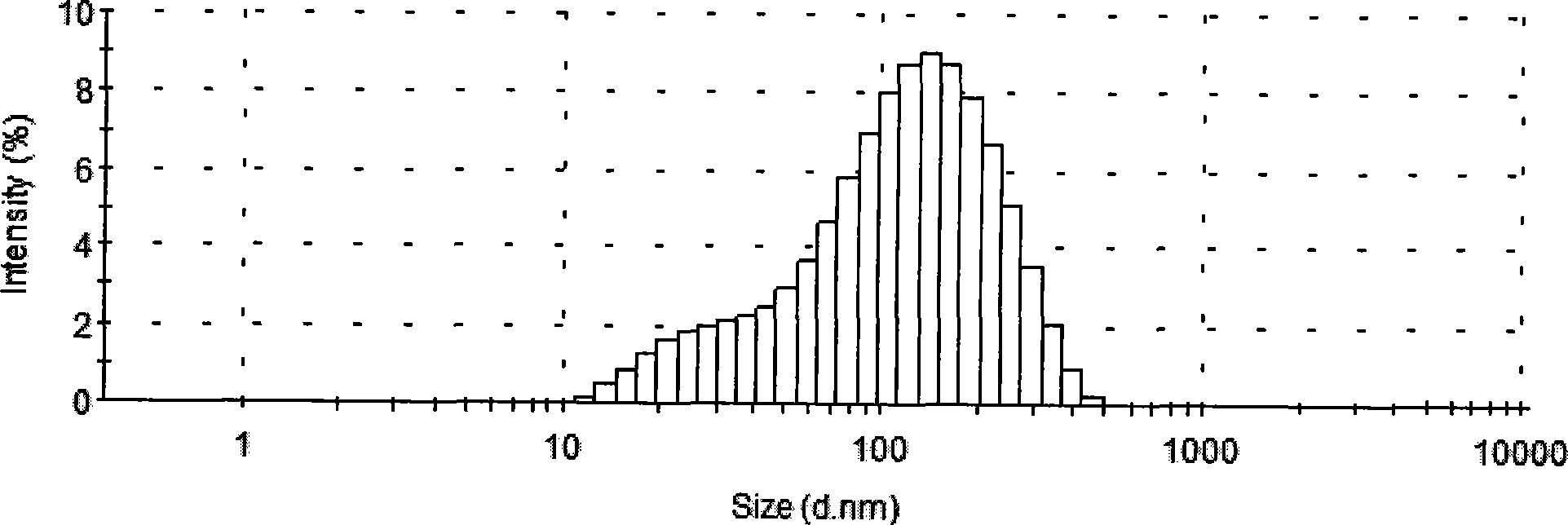
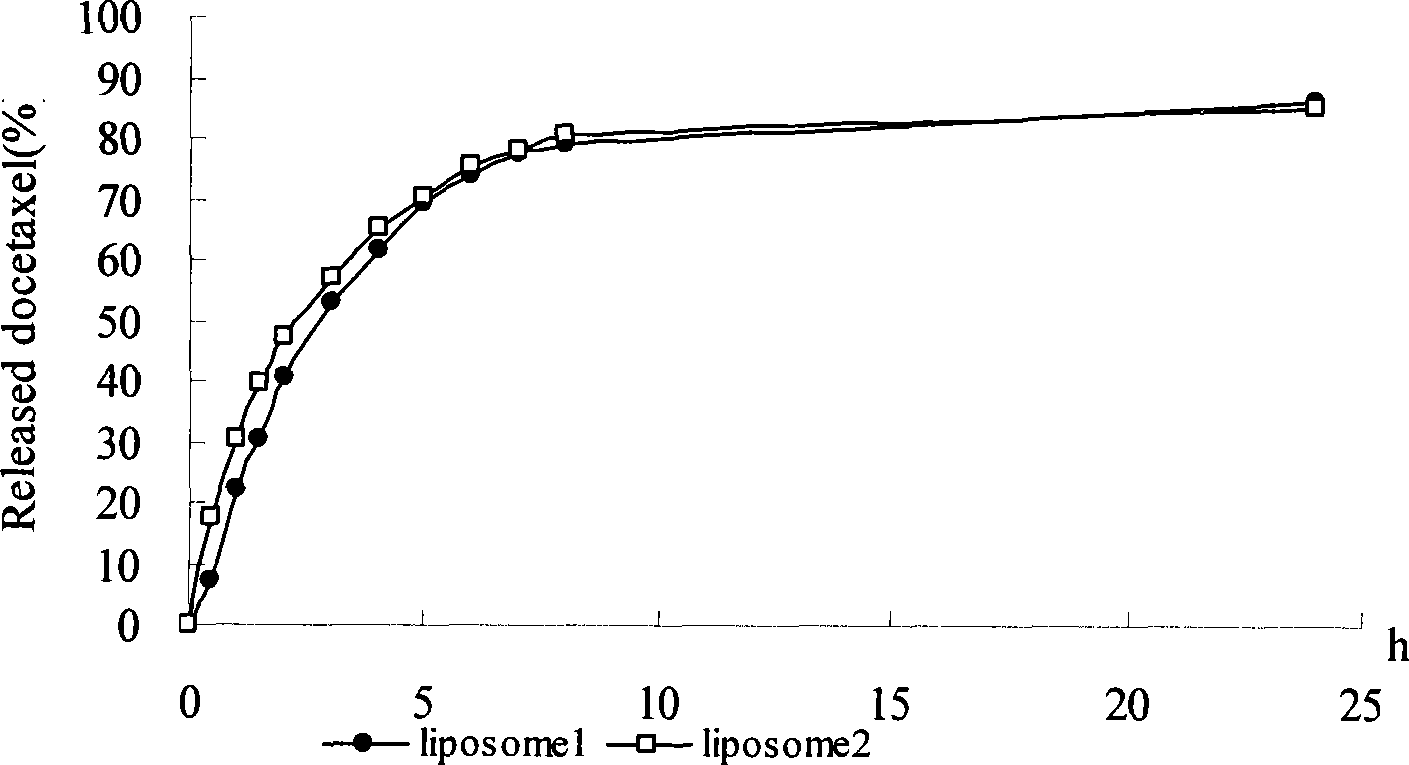
[0040] Weigh 25g of soybean lecithin (SPC), 1.5g of DSPE-PEG2000, 2.4g of cholesterol and 0.1g of α-tocopherol and dissolve them in 20mL of 40-45°C absolute ethanol solution, and quickly add the dissolved lipid solution to a constant temperature (40- 45°C) of 10mM phosphate buffer 500mL (pH6.5), and continue to stir for 1h to form a liposome suspension, add 1g of docetaxel, stir at room temperature for 2h, and homogenize through a high-pressure homogenizer to 80±10nm. Use polysulfone vacuum fiber ultrafilter for membrane diafiltration, exchange 8 volumes of sucrose solution to remove residual ethanol, keep the temperature of the working stream at 20-30°C, use 10% sucrose-10mM phosphate solution to volume and adjust docetaxel The concentration is 2mg / mL, sterilized by filtration through a 0.22μm microporous membrane, and the finished product can be obtained by subpackaging. The finished product can be stored at 2°C-8°C or freeze-dried until use.
Embodiment 2
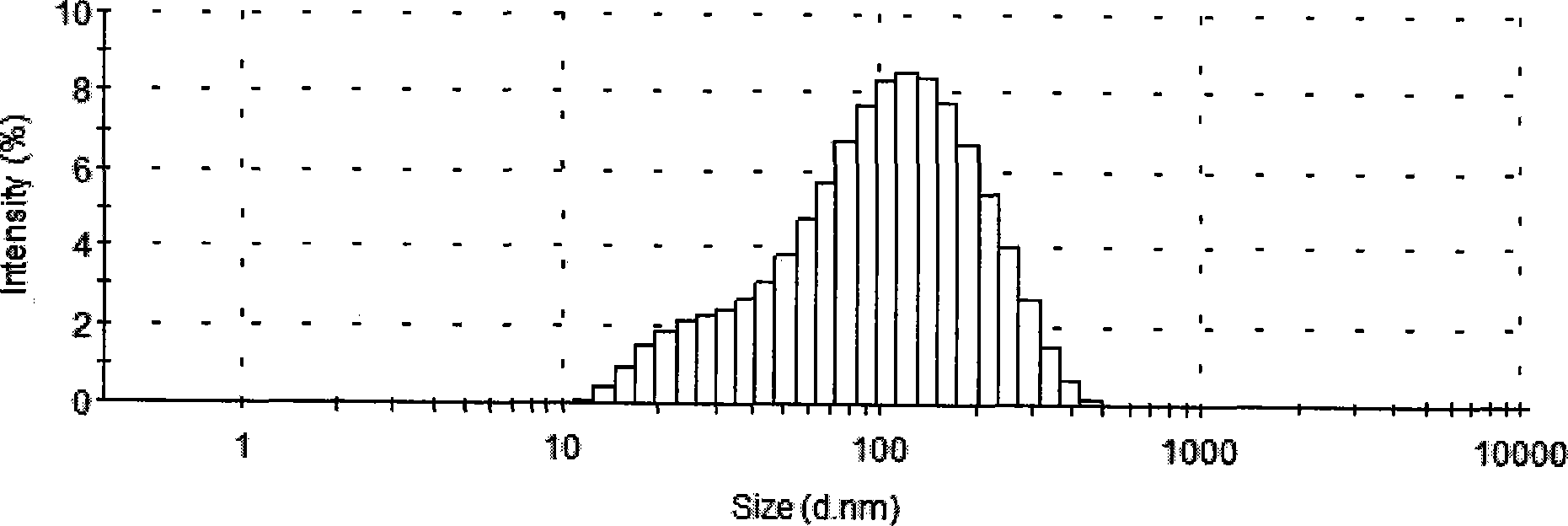
[0042] Weigh 30 g of egg yolk phospholipid (EPC), 1.5 g of egg yolk phosphatidylglycerol (EPG) and 0.12 g of α-tocopherol, add them to 500 mL of absolute ethanol, mix ultrasonically until dissolved, remove the organic solvent by spray drying, and use the obtained Place the lipid powder in a vacuum oven at 35°C overnight; prepare a 20mM sodium succinate solution, dissolve sucrose in the sodium succinate solution as a hydration solution, and adjust the pH value to 5.5 with an appropriate amount of succinic acid, water Multilamellar liposome suspension at a temperature of 40°C; add 1.5 g of micronized docetaxel, continue stirring for 2 h, homogenize with a high-pressure homogenizer until the average particle size is 120±10 nm, and set Contain and adjust the concentration of docetaxel to 3 mg / mL, filter and sterilize through a 0.22 μm microporous membrane, and then aliquot to obtain the finished product. The finished product can be stored at 2°C-8°C or freeze-dried until use.
Embodiment 2
[0043] Comparative example 2 common preparation method
[0044] Select egg yolk phospholipid (EPC) 30g, egg yolk phosphatidylglycerol (EPG) 1.5g and α-tocopherol 0.12g, be dissolved in chloroform-methanol (2: 1) solution and mix uniformly; The organic solvent is removed by vacuum evaporation method, Form lipid mixture; prepare 20mM sodium succinate solution, dissolve sucrose in sodium succinate solution as hydration solution, adjust pH to 5.5 with appropriate amount of succinic acid, hydration temperature is 40°C, mix multilamellar liposomes Suspension; after complete hydration, use a high-pressure homogenizer to homogenize to an average particle size of 120±10nm, dilute to volume with hydration solution and adjust to a docetaxel concentration of 3mg / mL, filter through a 0.22μm microporous membrane to remove The finished product can be stored at 2°C-8°C or freeze-dried until use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com