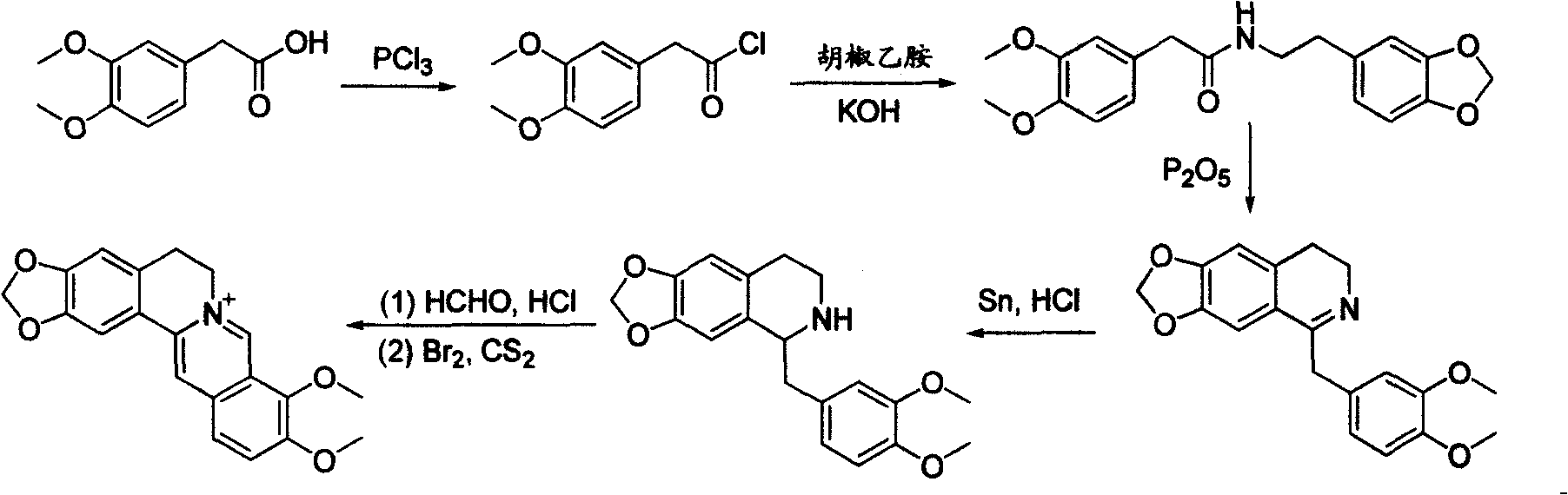
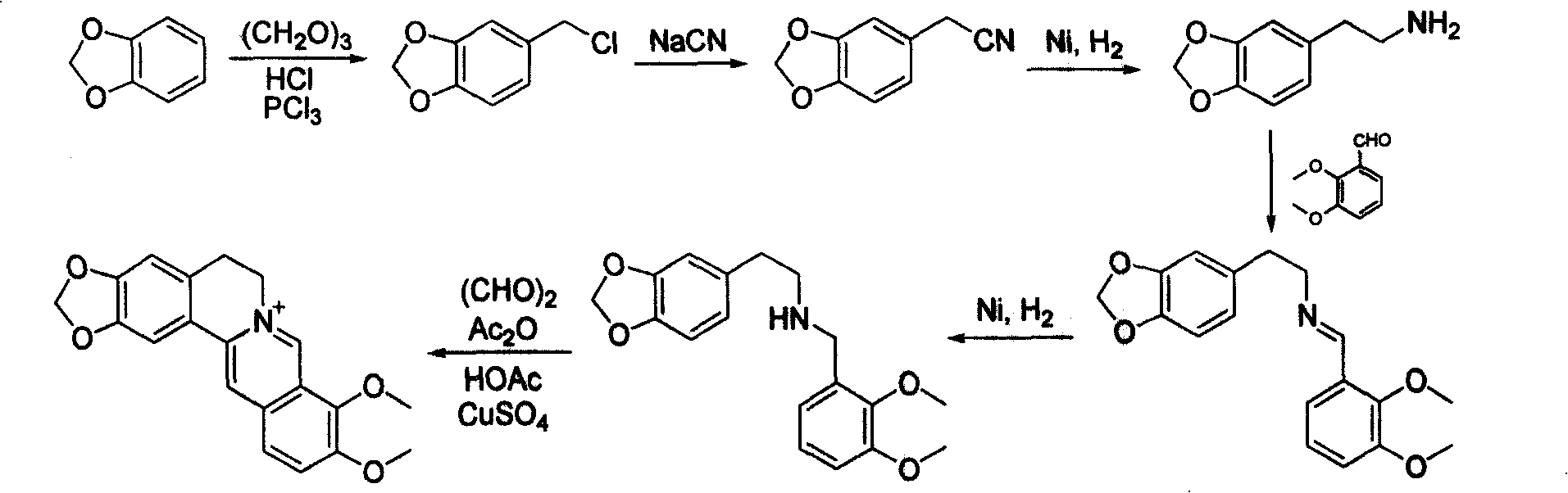
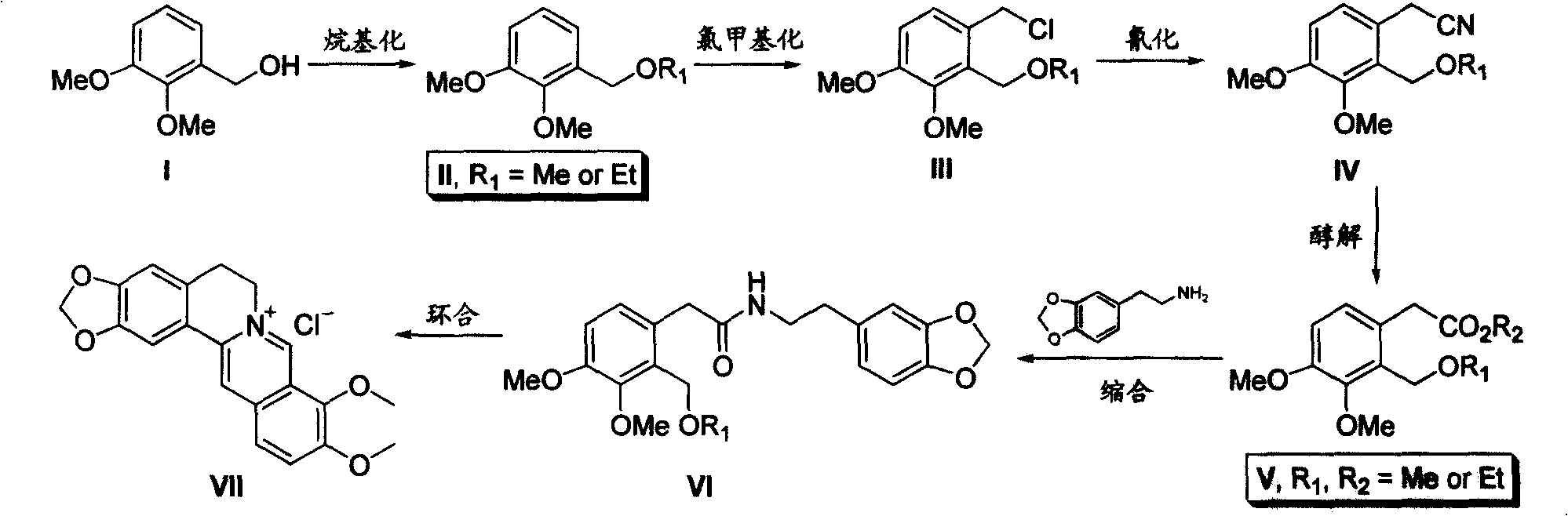
Process for producing hydrochloric berberine
A technology of berberine hydrochloride and dimethoxyphenylacetate is applied in the field of preparation of broad-spectrum antibiotic berberine hydrochloride, which can solve the problems of inconvenient operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of 1-(methoxymethyl)-2,3-dimethoxybenzene (II)
[0020] 168 grams of 2,3-dimethoxybenzyl alcohol (I) was dissolved in 350 mL of dichloromethane, 150 mL of 30% aqueous sodium hydroxide solution was added, and 138 grams of dimethyl sulfate was added dropwise at 80°C. After the dropwise addition, continue to stir the reaction at 80° C. until the raw material point disappears. The water phase was separated, and the organic phase was washed with 150 mL of water and 150 mL of 10% hydrochloric acid respectively. After drying, the solvent was evaporated to obtain 178 grams of oily 1-(methoxymethyl)-2,3-dimethoxybenzene , yield 98%. The oily product was directly used in the next reaction.
Embodiment 2
[0021] Embodiment 2: the preparation of 2-(methoxymethyl)-3,4-dimethoxybenzyl chloride (III)
[0022] 182 g of 1-(methoxymethyl)-2,3-dimethoxybenzene (II) was dissolved in 400 mL of toluene, and 200 mL of hydrochloric acid solution in which 33 g of paraformaldehyde was dissolved was added dropwise at 30°C. After the dropwise addition, keep the solution at 30°C, and continue to stir the reaction until the raw material point disappears. The water phase was separated, and the organic phase was washed with 150 mL of water and 150 mL of saturated sodium bicarbonate solution respectively. After drying, the solvent was evaporated to obtain oily 2-(methoxymethyl)-3,4-dimethoxychloride 210 grams of benzyl, yield 91%. The oily product was directly used in the next reaction.
Embodiment 3
[0023] Embodiment 3: the preparation of 2-(methoxymethyl)-3,4-dimethoxyphenylacetonitrile (IV)
[0024] 230 grams of 2-(methoxymethyl)-3,4-dimethoxybenzyl chloride (III) was dissolved in 500 mL of toluene, 200 mL of water and 75 grams of sodium cyanide were added, and 32 grams of tetrabutyl ammonium bromide. At 100 °C, the reaction was stirred until the starting point disappeared. The water phase was separated, and the organic phase was washed with 150 mL of water and 150 mL of saturated sodium bicarbonate solution, dried, and evaporated to dryness, and the crude product obtained was recrystallized with ethanol to obtain 2-(methoxymethyl)-3,4-bis 210 g of methoxyphenylacetonitrile, melting point 52-54°C, yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com