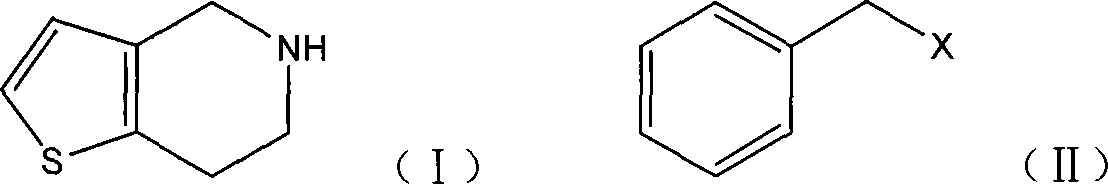Medicine midbody and preparation method thereof
An intermediate and pharmaceutical technology, applied in the field of pharmaceutical intermediates and their preparation, can solve the problems of large-scale production danger, harsh reaction conditions, flammability and explosion of n-butyllithium, etc. Excellent yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
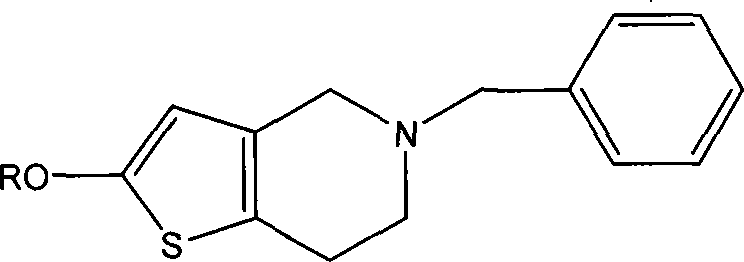
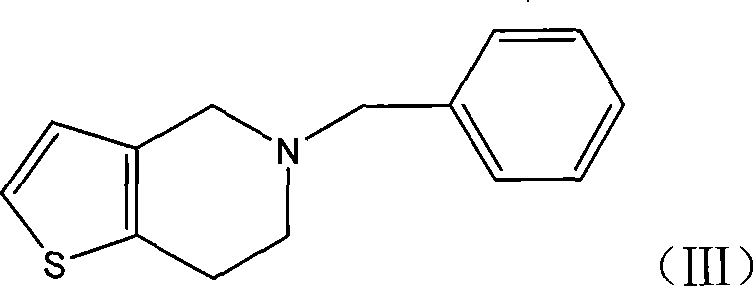
[0033] Preparation of 5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (formula III):
[0034] 4,5,6,7-tetrahydrothieno[3,2-c]pyridine (7.0g), potassium carbonate (7.2g) and acetonitrile (50ml) were mixed, benzyl chloride (6.1g) was added, stirred for 0.5 After 3 hours it was refluxed for 3 hours. Cool, filter, concentrate the filtrate to dryness, add ethyl acetate (30ml) and water (50ml), separate the layers, extract the aqueous layer with ethyl acetate (30ml×2), combine the organic layers, wash with water, dry, and concentrate to dryness to obtain 8.3 g of the title compound 5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine, yield 90.8%.
[0035] 4,5,6,7-tetrahydrothieno[3,2-c]pyridine (18.0g), potassium carbonate (18.6g), benzyl chloride (15.0g), sodium iodide (0.8g) in DMF ( 100ml) was stirred for 0.5 hours, then reacted at 80°C for 3 hours, cooled, added water (150ml) and ethyl acetate (100ml), separated, and the aqueous layer was extracted with ethyl acetate (50ml×2). ...
Embodiment 3
[0037] Preparation of 2-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (formula IV):
[0038] The compound (23.2g) obtained in Example 2 was dissolved in acetic acid (100.0ml), 40% hydrobromic acid (75.0ml), methanol (100ml), and the methanol of 30% hydrogen peroxide (33.0ml) was added dropwise under ice-water bath cooling (100ml) solution, stirred at room temperature for 3 hours. Sodium thiosulfate solution (150ml) was added dropwise, then saturated sodium carbonate solution was added dropwise until the pH was 9, extracted with dichloromethane (100ml×3), the organic layers were combined, washed with water, dried, and concentrated to dryness to obtain 30.5g of light yellow solid , yield 97.8%.
Embodiment 4~6
[0040] Preparation of 2-methoxy-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (formula V):
[0041] Dissolve sodium (0.43g) in methanol (20ml), add 2-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (3.88g), bromide Cuprous (0.17g), stirred and refluxed for 12 hours. Cool, filter, concentrate the filtrate to dryness, add ethyl acetate (30ml) and water (50ml), separate the layers, extract the aqueous layer with ethyl acetate (30ml×2), combine the organic layers, wash with water, dry, and concentrate to dryness to obtain 3.3 g of oily matter was separated by column to obtain 1.3 g of the title compound, with a yield of 39.8%.
[0042] Dissolve sodium (5.6g) in methanol (120ml), concentrate to dryness, add tetrahydrofuran (100ml), 2-bromo-5-benzyl-4,5,6,7-tetrahydrothieno[3,2-c ] Pyridine (7.5g), cuprous bromide (0.34g) and sodium iodide (0.15g), stirred and refluxed for 24 hours. Cool, filter and wash with ethyl acetate, concentrate the filtrate to dryness, add ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com