Long-acting medicine-loading orlon fibre capable of degrading partly, preparation and application thereof
A technology of polyacrylonitrile fiber and polyacrylonitrile, which is applied in the field of long-acting drug-loaded polyacrylonitrile fiber, can solve the problems of drug loss and incomplete drug-loaded drug release, so as to improve long-term controlled release performance and drug-loading performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: PCL / PAN drug-loaded fiber is prepared by wet spinning
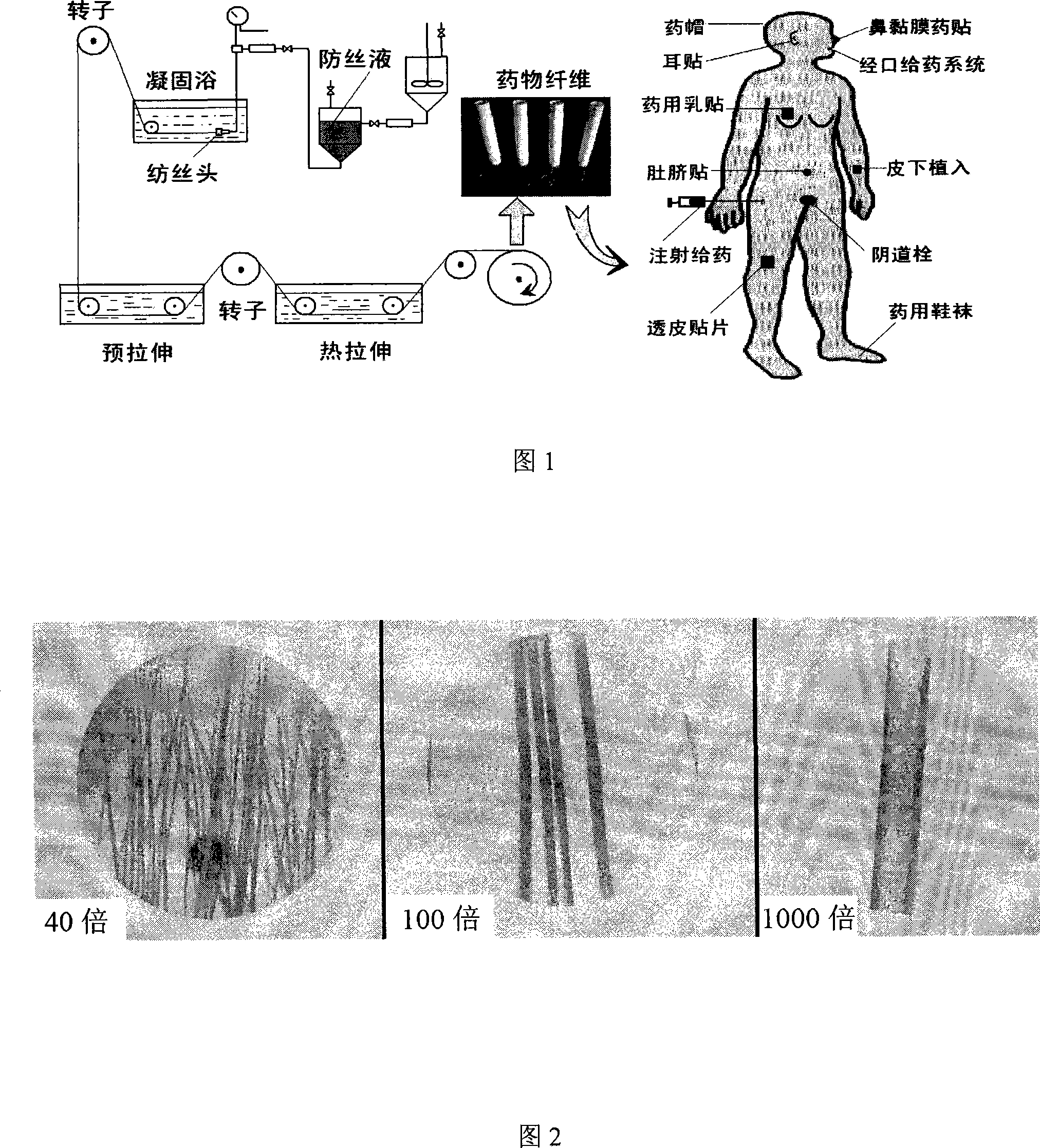
[0028] The fiber spinning solution is prepared according to the following steps: first, 10.0g drug ibuprofen and 1.0g transdermal penetration enhancer borneol are dissolved in 350mL N, N-dimethylacetamide (DMAc) at normal temperature, and then 20.0 Disperse gε-polycaprolactone (PCL), 70.0g polyacrylonitrile (PAN) fine powder in the drug solution, and finally place it in a water bath at 80°C for 24 hours under stirring conditions, and then obtain the spinning stock solution after cooling to room temperature . The drug-loaded fiber was prepared by wet method (as shown in Figure 1), dry method, dry-wet method, electrospinning, etc., and 40% DMAc aqueous solution was used as the coagulation liquid, and the nanometer drug-loaded fiber was observed with a polarizing microscope, as shown in Figure 2. Using a reading microscope, the fiber diameter was 123±16 μm.
Embodiment 2
[0029] Embodiment 2: Dry spinning prepares PCL / PAN drug-loaded fiber
[0030] The spinning dope was prepared according to Example 1, and the drug-loaded fiber was prepared by a dry process.
Embodiment 3
[0031] Embodiment 3: Preparation of PCL / PAN drug-loaded fiber by dry-wet spinning
[0032] The spinning dope was prepared according to Example 1, and the drug-loaded fiber was prepared by a dry-wet process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com