Preparation of taurine and derivatives thereof
A kind of derivative, the technology of taurine, applied in the field of preparation of taurine and derivatives thereof, can solve the problems such as unfavorable industrial production, complicated operation and handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
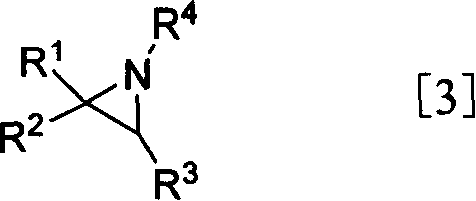
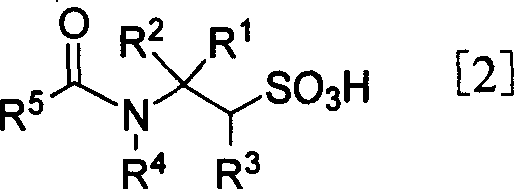
[0058] Preparation of N-acetyltaurine and taurine (1a)
[0059] (1) Open loop:
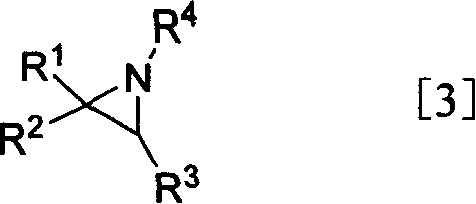
[0060] Add 1.332g (31mmol) aziridine, 5.89g (77.4mmol) thioacetic acid and 150mL tetrahydrofuran into a 250mL three-necked flask, stir at reflux for 8h, spin off the solvent to obtain an oily product.
[0061] (2) Oxidation:
[0062] The oil obtained above was dissolved in 88% HCO 2 H (27 mL). 30% H 2 o 2 (45mL) and 88% HCO 2 H (150 mL) was mixed and stirred at room temperature for 1 h, and added dropwise to the above solution under cooling in an ice-water bath, keeping the reaction temperature at 0-5°C. The reaction mixture was brought to room temperature and stirred for one day. The solvent was distilled off to obtain the solid product N-acetyltaurine, and the total yield of the two steps was 98%.
[0063] (3) Hydrolysis:
[0064] The above solid product was dissolved in 10% hydrochloric acid and refluxed overnight. The solvent was evaporated, and the residue was washed with ethanol to...
Embodiment 2
[0066] Preparation of (S)-3-methyl-2-acetylamino-butylsulfonic acid and (S)-3-methyl 2-amino-butylsulfonic acid (1b)
[0067] According to the method described in Example 1, (S)-2-isopropylaziridine was used as a raw material to obtain (S)-3-methyl-2-acetylamino-butylsulfonic acid, colorless crystals, two The total yield in one step is 65%; its hydrolysis gives (S)-3-methyl-2-amino-butylsulfonic acid as colorless crystals, the melting point is 326-329°C, and the total yield in three steps is 61%.
Embodiment 3
[0069] Preparation of (S)-3-methyl 2-amino-butylsulfonic acid (1b)
[0070] According to the method described in Example 1, ring-opening with thioacetic acid in benzene, using (S)-2-isopropylaziridine as a raw material to obtain (S)-3-methyl-2-amino-butyl Sulfuric acid, colorless crystals, melting point 326-329°C, the total yield of three steps is 70%. 13 C NMR (75.5MHz, HCO 2 H)δ: 16.2, 17.1, 30.3, 49.3, 54.6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com