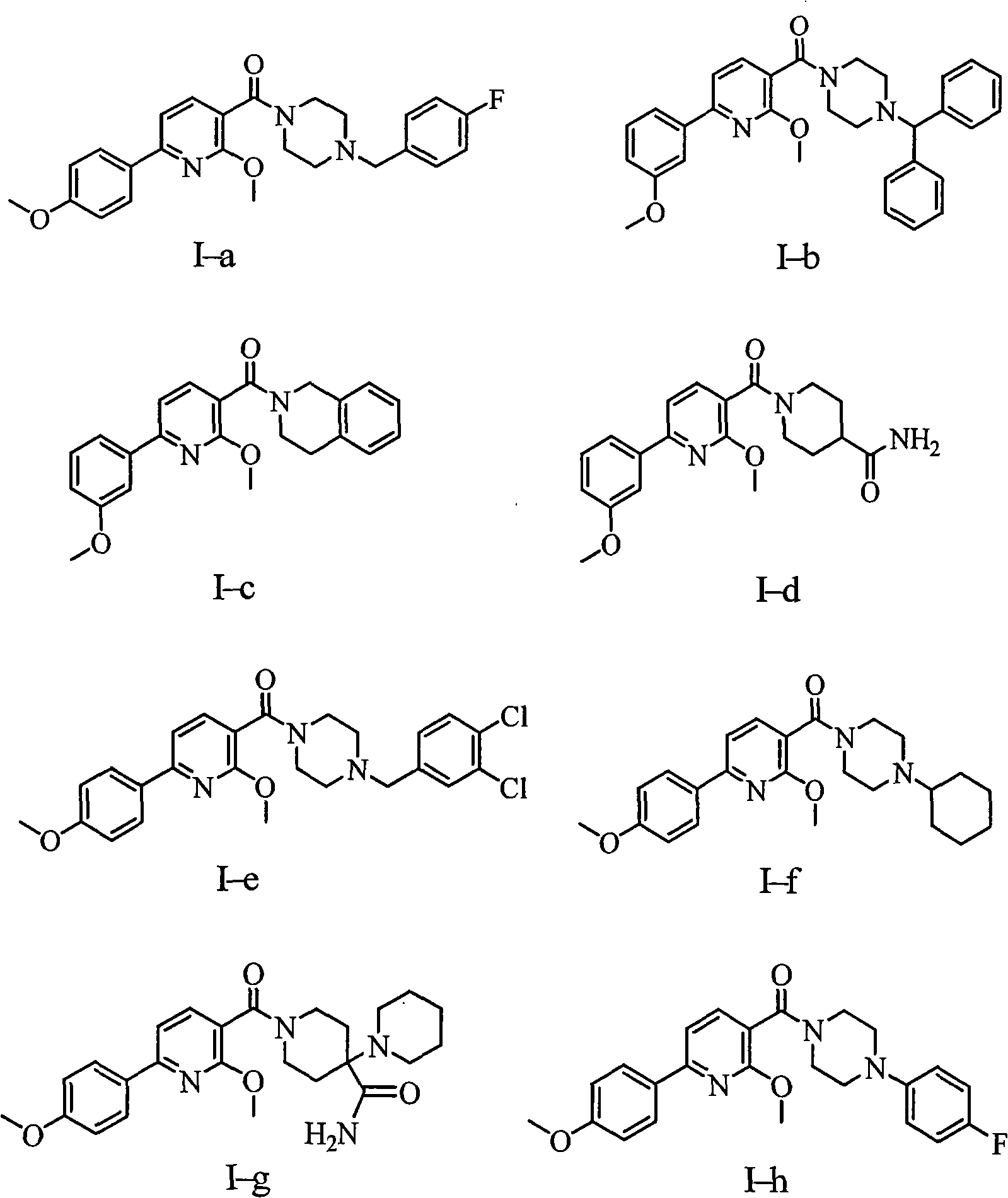
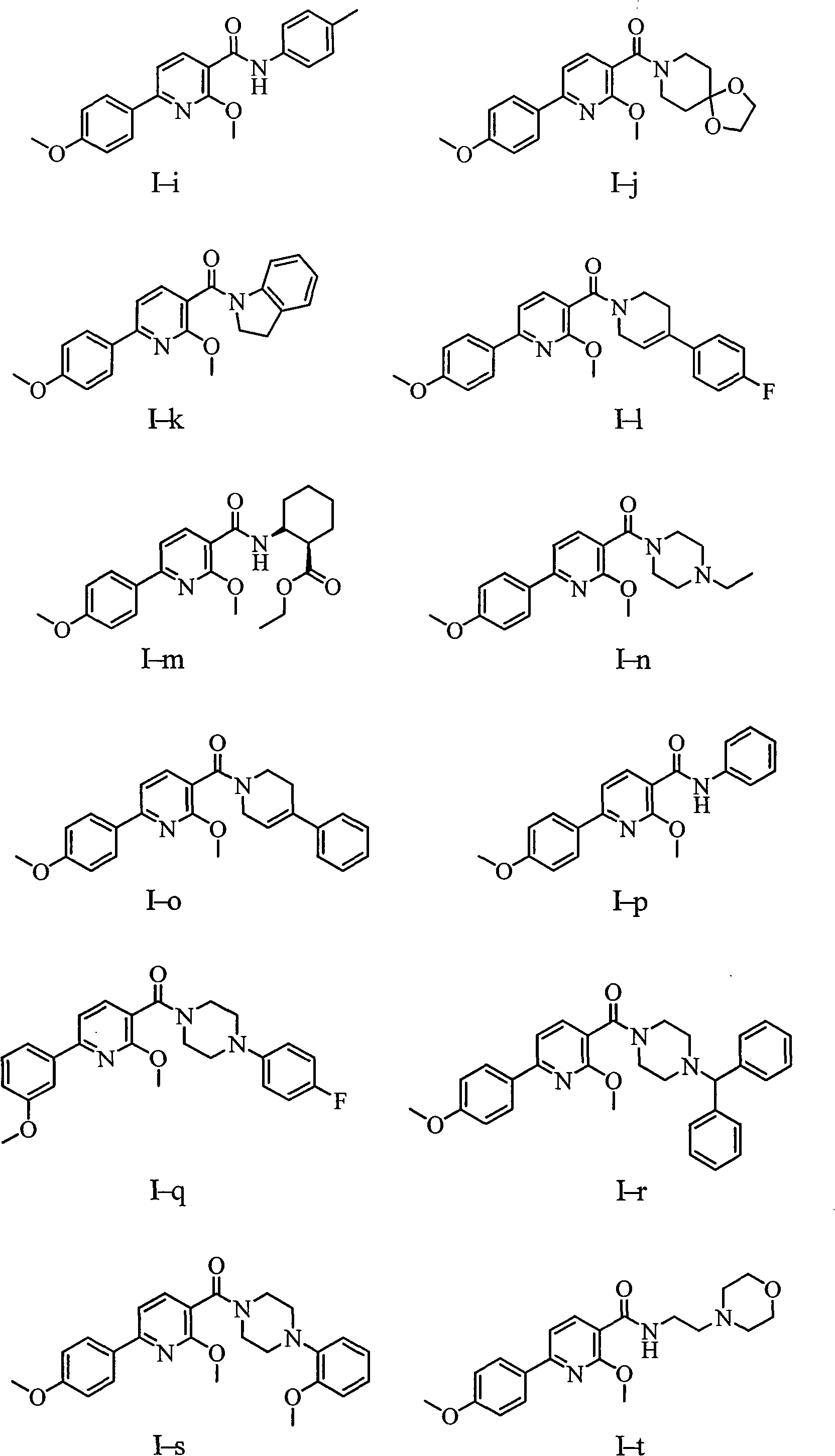
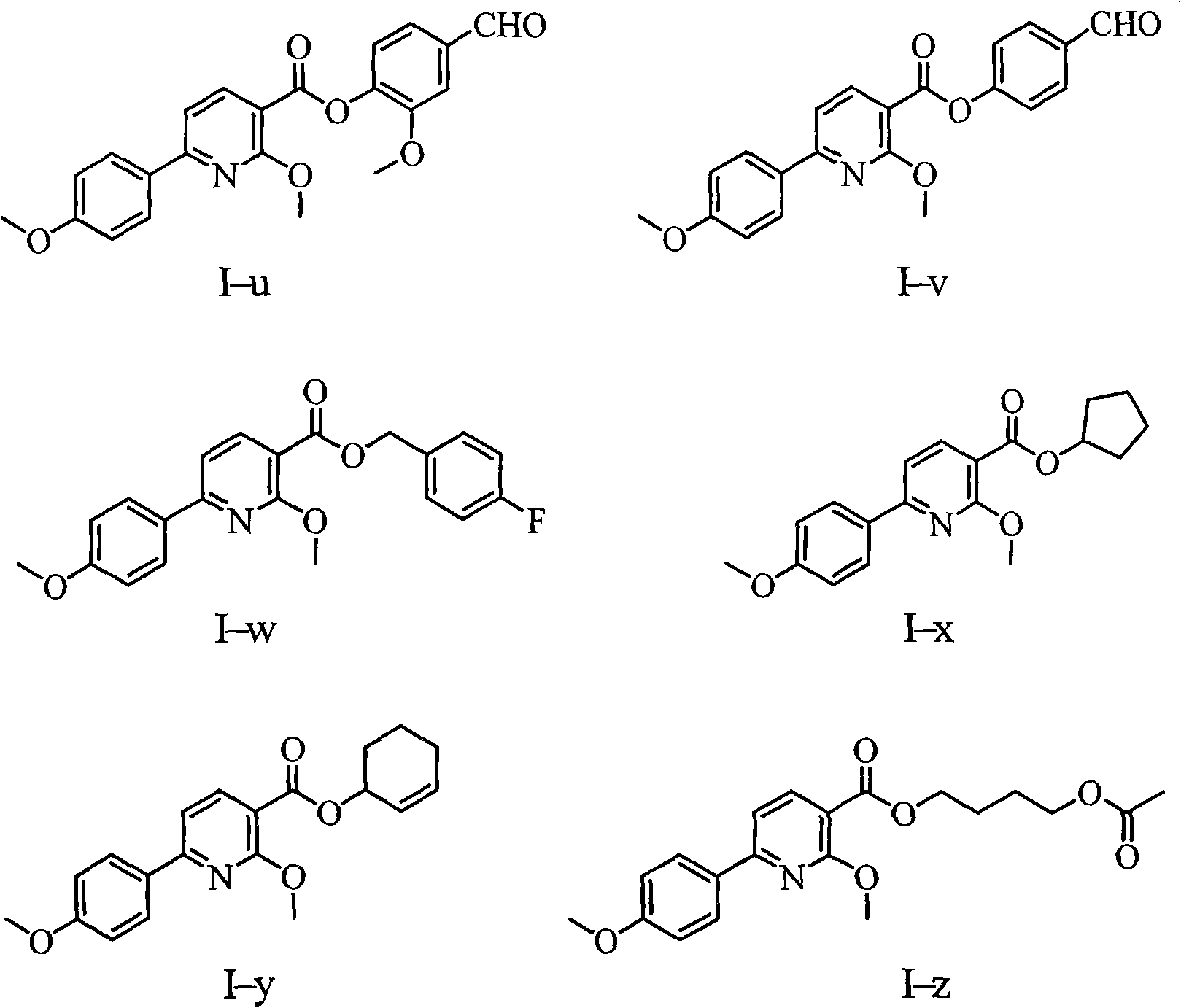
Aryl pyridine compounds and medicament uses thereof
A compound and aryl technology, applied in the field of 6-aryl-3-substituted-pyridone derivatives and their preparation, can solve the problems of weakened signal transmission, decreased concentration of acetylcholine, disordered synthesis and secretion of acetylcholine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044] Preparation example 1: Preparation of starting compound IIa (p-methoxyacetophenone):
[0045] Compound IIa
[0046] Methoxybenzene (10.8 g, 0.1 mol) was dissolved in 150 ml of dichloromethane, then anhydrous zinc chloride powder (26.8 g, 0.20 mol) was added, and acetic anhydride (15.3 g, 0.15 moles); after the dropwise addition, the reaction was slowly raised to room temperature for 7 hours, then the reactant was carefully poured into 600 ml of ice water, and extracted 3 times with ethyl acetate; the organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated The crude product was obtained as a colorless oil, and the starting compound IIa (p-methoxyacetophenone) (13.1 g, yield 87%) was obtained by short silica gel column chromatography. White solid, melting point: 35-38°C. H NMR spectrum 1 H-NMR (400MHz, deuterated chloroform, δppm) 2.56 (single peak, 3H, COCH 3 ), 3.87 (single peak, 3H, OCH 3 ), 6.93 (doublet, 2H, J=8.4Hz, H-3,5), 7.94 (...
preparation example 2
[0047] Preparation example 2: Preparation of starting compound IIb (m-methoxyacetophenone):
[0048] Compound IIb
[0049] 3-Hydroxyacetophenone (13.6 grams, 0.1 moles) was dissolved in 150 milliliters of acetone, 20 grams of potassium carbonate (0.15 moles) and dimethyl sulfate (12.6 grams, 0.1 moles) were added; reflux reaction for 10 hours, TLC showed After the reaction was complete, filter, wash the filter cake with ethyl acetate, and concentrate to obtain a crude brown oil, which was subjected to short silica gel column chromatography to obtain Compound IIb (m-methoxyacetophenone), 12.1 g, with a yield of 81%. Colorless oil.
preparation example 3
[0050] Preparation example 3: Preparation of intermediate compound IIIa [3-cyano-6-(4-methoxyphenyl)-2H-pyridin-2-one]:
[0051] Compound IIIa
[0052] Sodium metal (2.76 g, 120 mmol) was added in 250 ml of ether, 1 ml of ethanol was added dropwise, compound IIa (p-methoxyacetophenone) (100 mmol) and ethyl formate ( 150 mmol) mixture, after the dropwise addition, the mixture was stirred for 15 minutes, then warmed up to room temperature and reacted for 1 hour, after diethyl ether was distilled off under reduced pressure, cyanoacetamide (12.6 grams, 150 mmol) and water (400 mmol) were added to the solid mixture. ml). After the mixture was refluxed for 8 hours, cooled, acidified with acetic acid, filtered to obtain a yellow solid, after drying, the initial product was recrystallized from ethanol to obtain intermediate compound IIIa[3-cyano-6-(4-methoxyphenyl) -2H-pyridin-2-one]: yield 56%, light yellow solid; melting point > 250 ° C; R f (Dichloromethane / methanol 20:1) 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com