Vaginal effervescence tablet composition containing solid lipid
A technology of solid lipids and effervescent tablets, which is applied to medical preparations containing active ingredients, drug combinations, and medical preparations with non-active ingredients, etc. It can solve the problem that the adhesion of effervescent tablets affects drug distribution, jelly Difficult to remove, uneven drug distribution and other problems, to achieve the effect of low viscosity, prolonged effect, and easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
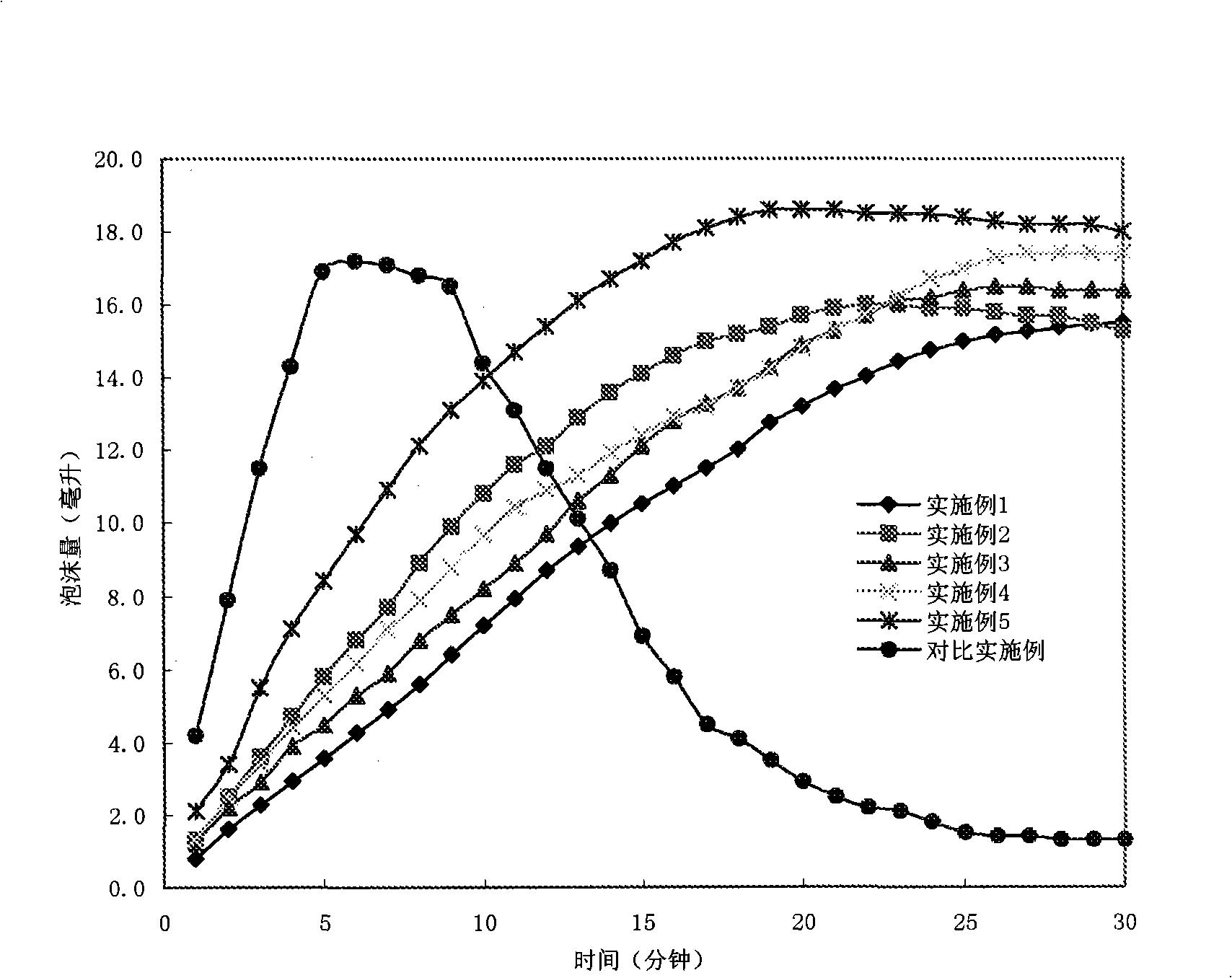
Image
Examples
Embodiment 1
[0102] Formula of Nystatin Vaginal Effervescent Tablet 1
[0103] Preparation Content of ingredients in each tablet (mg)
[0104] Nystatin 20
[0105] 888 ATO 95
[0106] Lactose 902
[0107] Sodium bicarbonate 73
[0108] Tartaric acid 67
[0109] Sodium Lauryl Sulfate 18
[0110] Di-tert-butyl-p-cresol 2
[0112] Preparation method: mix nystatin, part of lactose, and di-tert-butyl-p-cresol, use absolute ethanol solution to make soft material, pass through 60 mesh to make wet granules, dry at 30°C for 60 minutes, and pass through 60 mesh for granulation. Add sodium lauryl sulfate, sodium bicarbonate, tartaric acid, 888ATO, magnesium stearate and remaining lactose are mixed evenly and compressed into diamond-shaped tablets. The hardness of gained tablet is controlled at 10-12kg / mm 2 .
[0113] The nystatin vaginal effervescent tablet that adopts embodiment 1 to prepare carries out quality research, and the results are as follows:
...
Embodiment 2
[0116] Formulation of Secnidazole Vaginal Effervescent Tablet 2
[0117] Preparation Content of ingredients in each tablet (mg)
[0118] Secnidazole 250
[0119] ATO 5 200
[0120] Lactose 540
[0121] Sodium bicarbonate 105
[0122] Citric acid 85
[0123] Sodium Lauryl Sulfate 20
[0125] Preparation method: secnidazole, ATO 5, lactose, sodium bicarbonate, citric acid, sodium lauryl sulfate and magnesium stearate were mixed in a container for 25 minutes, then transferred to a tablet machine and compressed into diamond-shaped tablets. The hardness of gained tablet is controlled at 9-11kg / mm 2 .
[0126] The obtained secnidazole vaginal effervescent tablet that adopts embodiment 2 to prepare carries out quality research, and the results are as follows:
[0127] project
Embodiment 3
[0129] Formulation of Clindamycin Phosphate Vaginal Effervescent Tablet 3
[0130] Preparation Content of ingredients in each tablet (mg)
[0131] Clindamycin Phosphate 100
[0132] 888 ATO 190
[0133] Lactose 510
[0134] Povidone K30 30
[0135] Sodium bicarbonate 80
[0136] Tartaric acid 66
[0137] Sodium Lauryl Sulfate 20
[0139] Preparation method: clindamycin phosphate, 888 ATO, part of lactose, tartaric acid, and sodium lauryl sulfate were mixed in a container for 25 minutes, and an appropriate amount of 5% povidone K30 aqueous solution was added to prepare a soft material, granulated through a 40-mesh sieve, and air-dried at 50 degrees for 45 minutes , after taking out, pass through a 40-mesh sieve to obtain granule I. In addition, put sodium bicarbonate and part of lactose in a container and mix for 25 minutes, add an appropriate amount of 5% povidone K30 aqueous solution to prepare a soft material, pass through a 40-mesh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com