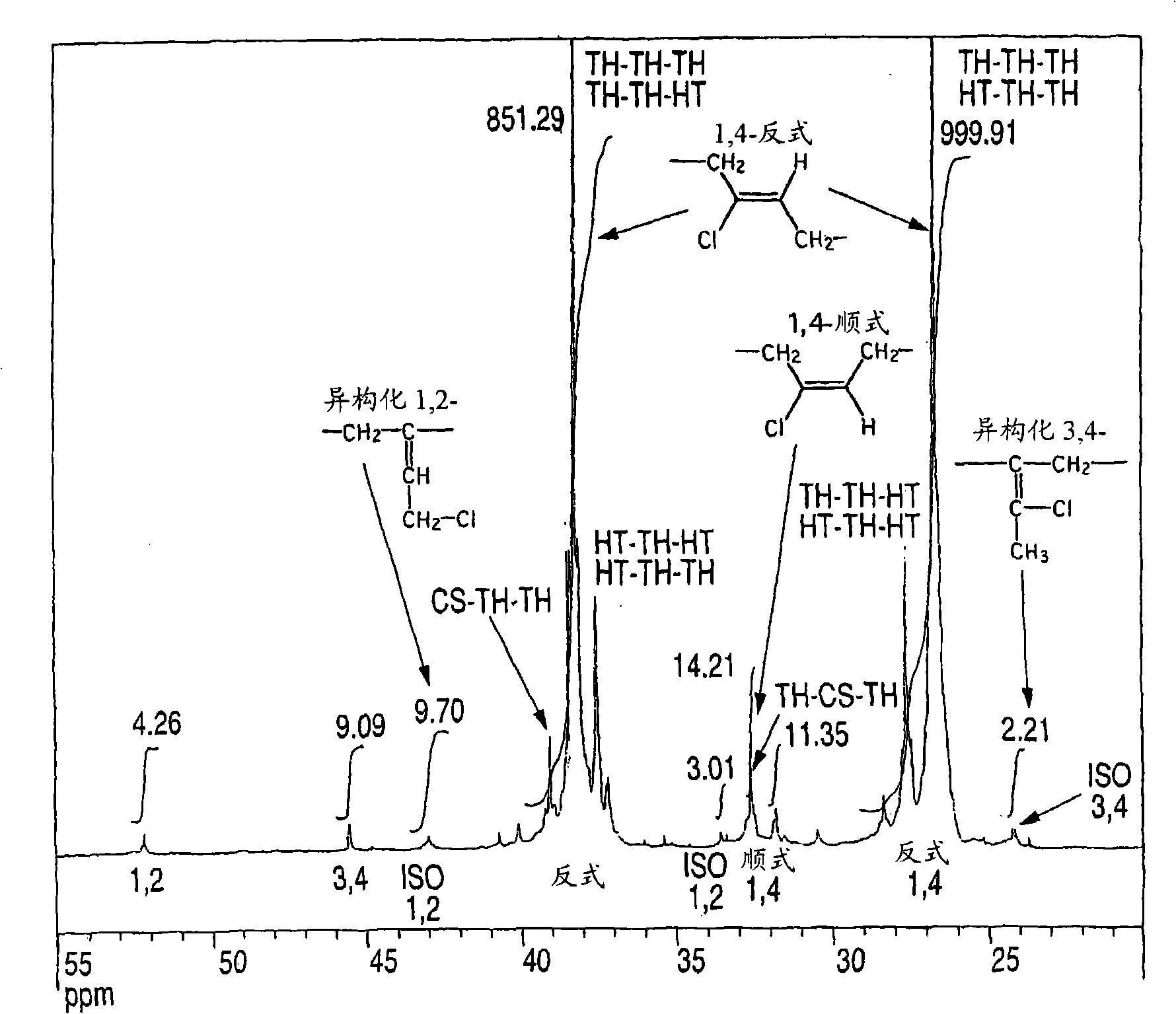
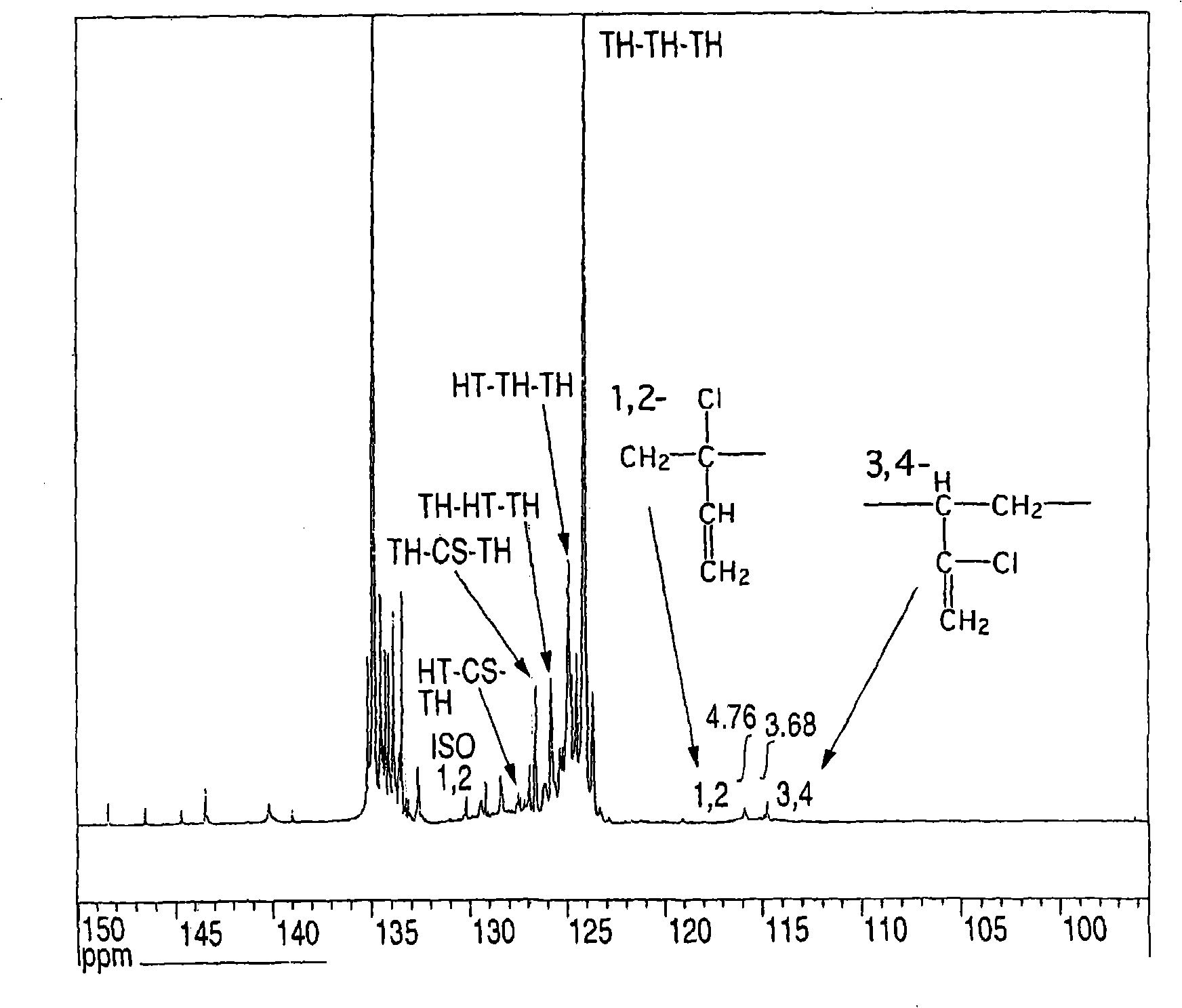
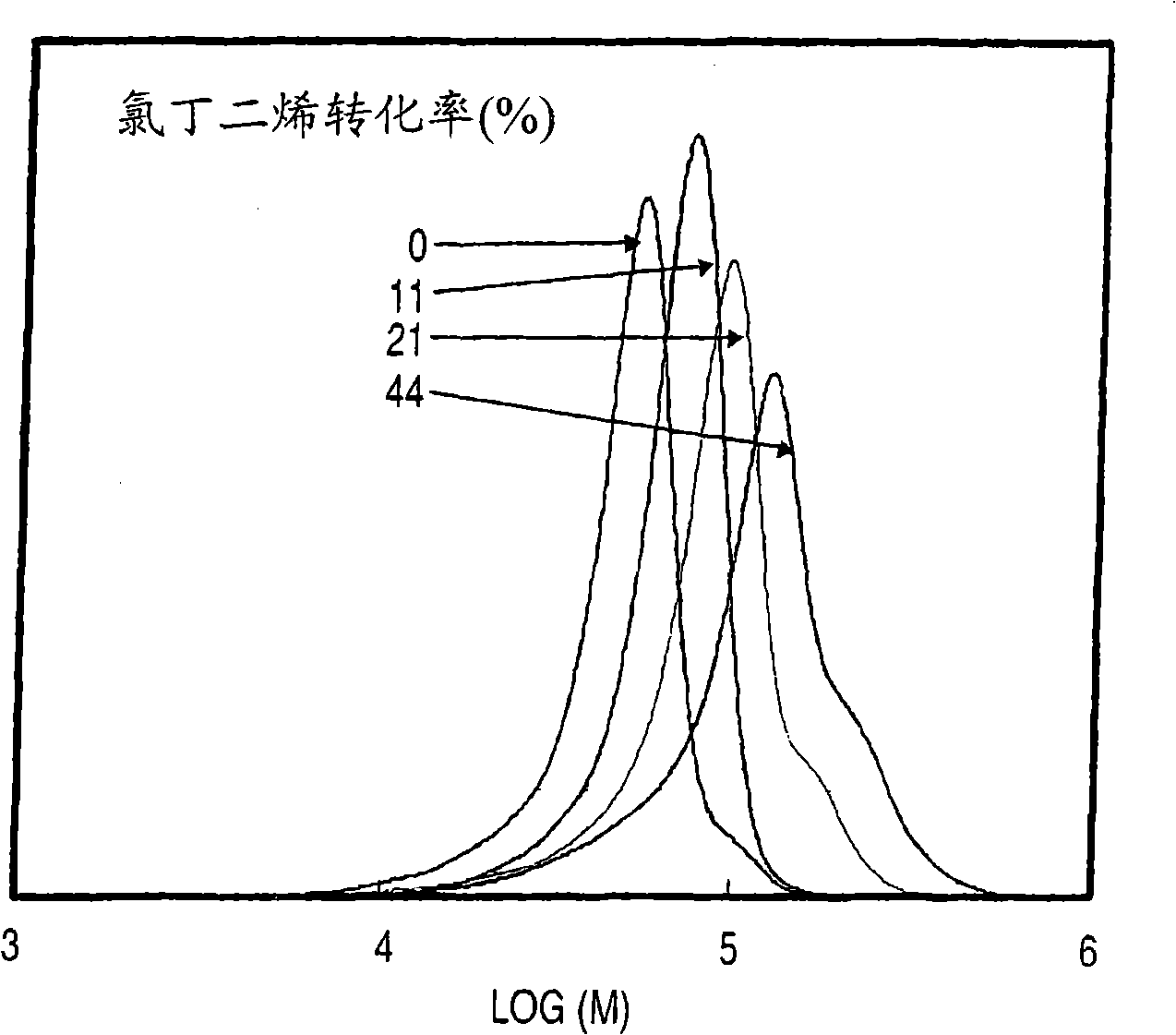
Chloroprene block copolymer and soapless polychloroprene latex, and processes for production of copolymer and latex
A technology of block copolymer and polychloroprene, which is applied in the direction of adhesives, etc., can solve the problem that there is no record of the physical properties of block copolymers, and achieve the effect of improving adhesion and improving adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0115] In order to describe the present invention more specifically, Examples are shown below, but the present invention is not limited to these Examples.
[0116] First, reference examples, synthesis examples 1 to 15, examples 1 to 26, and comparative examples 1 to 5 are shown regarding the chloroprene-based block copolymer of the present invention. In addition, the values in these examples were measured by the following methods.
[0117]
[0118] The conversion rate of the monomer during the polymerization was calculated using Shimadzu Gas Chromatography GC-17A (capillary column NEUTRABOND-5 manufactured by GL Science, hydrogen flame ionization detector), using benzene as an internal standard. .
[0119]
[0120] The number average molecular weight Mn, the weight average molecular weight Mw and the molecular weight distribution Mw / Mn of the polymer are measured under the following conditions using GPC8220 manufactured by Tosoh (Tosoh One) Co., Ltd. (eluent=tetrahydrof...
Synthetic example 1
[0142]Add 0.30 g of carbamate represented by the following general formula (7), 30.0 g of styrene, 4.0 g of acrylonitrile, and 20.0 g of methyl ethyl ketone into a 200 ml Pyrex heat-resistant glass (registered trademark) flask equipped with a nitrogen inlet tube , and then repeated freezing-degassing-melting 3 times, after fully degassing, stirring under a nitrogen atmosphere, and irradiating ultraviolet light (manufactured by Ushio Electric Co., Ltd., UM452( 450W)), while carrying out 20 hours of polymerization. The polymerization conversions of styrene and acrylonitrile at this time were 30% and 57%. The contents were poured into a large amount of methanol to precipitate a polystyrene / acrylonitrile copolymer to obtain a polymer (A). The number average molecular weight Mn measured by GPC was 14600, the weight average molecular weight Mw was 29100, and the molecular weight distribution Mw / Mn was 1.99. The sulfur content in the polymer was 0.66% by weight.
[0143] [chemical...
Synthetic example 2
[0146] Add 0.30 g of carbamate represented by general formula (7) and 0.14 g of carbamic acid disulfide represented by following general formula (8) in a 200 ml Pyrex heat-resistant glass (registered trademark) flask equipped with a nitrogen inlet tube. material, 30.0g styrene, 5.0g acrylonitrile, 20.0g methyl ethyl ketone, and then repeated freezing-degassing-melting three times, after fully degassing, stirring under nitrogen atmosphere, and from 80mm Polymerization was performed for 20 hours while irradiating ultraviolet rays (manufactured by Ushio Electric Co., Ltd., UM452 (450W)) at a distance of 20 Å. The polymerization conversions of styrene and acrylonitrile at this time were 29% and 56%. The contents were poured into a large amount of methanol to precipitate a polystyrene / acrylonitrile copolymer to obtain a polymer (A). The number average molecular weight Mn measured by GPC was 13100, the weight average molecular weight Mw was 25900, and the molecular weight distribut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adhesive strength | aaaaa | aaaaa |
| Peel strength | aaaaa | aaaaa |
| Peel strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com