Colibacillus for recombining L-arabinose isomerase and method for preparing tagatose
A technology of arabinose and Escherichia coli, applied in the field of L-arabinose isomerase converting tagatose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The construction of recombinant Escherichia coli was carried out as described in "Molecular Cloning Experiment Guide" (Third Edition) (Sambrook et al., Science Press, 2002). According to the araA gene sequence (Genebank No. 947511) in Escherichia coli published by Genebank, the primers were designed as follows:
[0038] 5' Primer (Seq ID No.1): 5'-C CCATGG GCATGACGATTTTTG(Ncol)
[0039] 3' Primer (Seq ID No.2): 5'-GGG TTCGAA TTAGCGACGAAAC (HindIII)
[0040] PCR was performed using the chromosomal DNA of Escherichia coli JM109 as a template: 95°C for 5 min, 30× (95°C for 40 s, 62°C for 40 s, 72°C for 1 min), 72°C for 10 min. The PCR product and the pET-28a vector were cut with restriction enzymes NcoI and HindIII and ligated with T4 DNA ligase, and then transformed into Escherichia coli BL21 (DE3) competent cells.
[0041] The transformant was smeared on LB solid medium containing kanamycin, and the single colony that grew out was selected, and the plasmid was extr...
Embodiment 2
[0043] Scrape a loop of E.coli BL21 / pET-araA from the slant and inoculate it in LB medium containing kanamycin, and culture it with shaking at 37°C for 16 hours. Draw 1mL of the bacterial liquid and inoculate it into a 250mL Erlenmeyer flask containing 30mL of LB+kanamycin medium, culture with shaking at 37°C for 3h, add 8mg / mL lactose as an inducer, continue to culture for 5h, and collect microbial cells by centrifugation at 10,000RPM.
[0044] Add about 0.2 g of the above wet bacteria into 30 mL of a solution containing D-galactose (50 g / L), shake and react at 37°C for 24 hours, sample and analyze, and obtain about 2.5 g / L D-tagatose. However, no D-tagatose was detected in the reaction solution of Escherichia coli containing empty vector.
Embodiment 3
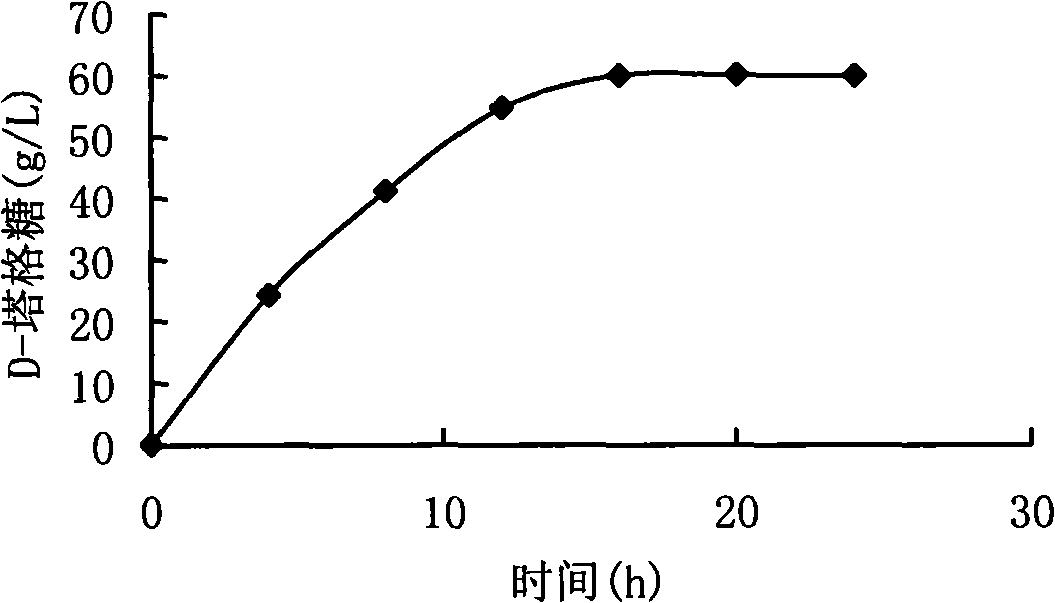
[0046] The preparation method of wet thalline is the same as that in Example 2. About 0.2g of wet bacteria was dissolved in 10mL of physiological saline, and the cell wall was broken by ultrasonic waves to obtain a crude enzyme solution. Add 20mL LD-galactose solution to make the final concentration 100g / L. The reaction was shaken at 37°C for 24 hours, and the amount of D-tagatose was analyzed every 4 hours. The results were as follows: figure 1 . After 16h, the amount of D-tagatose stabilized at 60.2g / L.
[0047] Example 3
[0048] The preparation of L-arabinose isomerase crude enzyme solution is as in Example 2.
[0049] Mix about 10 mL of this enzyme solution with an equal volume of 5% (w / w) sodium alginate solution, then drop into 1% (w / w) calcium chloride solution, stir well, put it in the refrigerator overnight, filter Take out the calcium alginate pellets for later use.
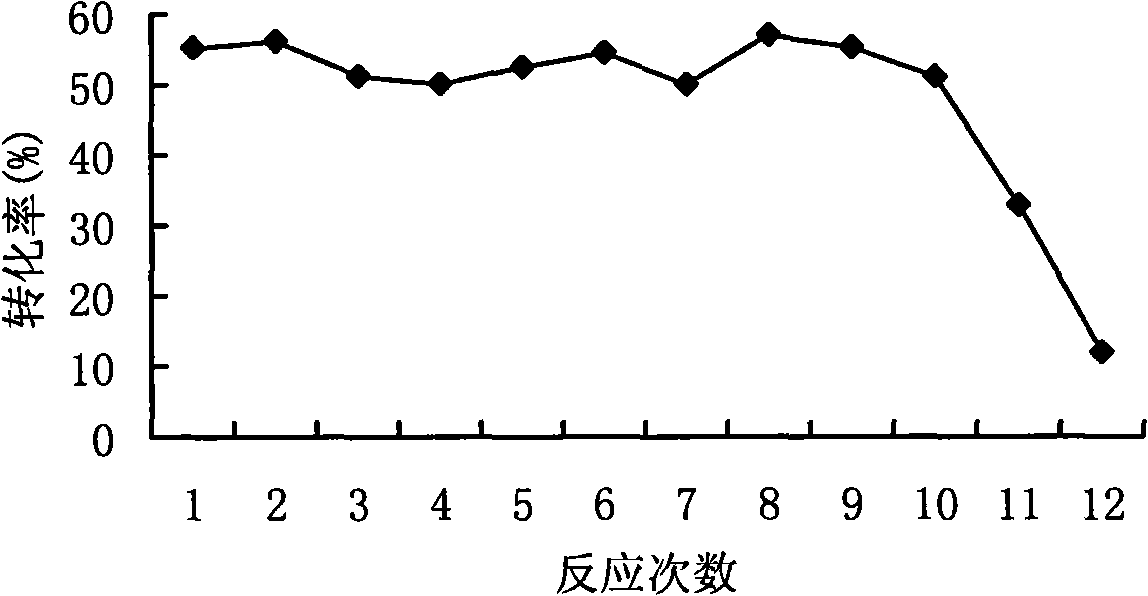
[0050] Add about 20 g of the above-mentioned immobilized L-arabinose isomerase to 20 mL of 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com