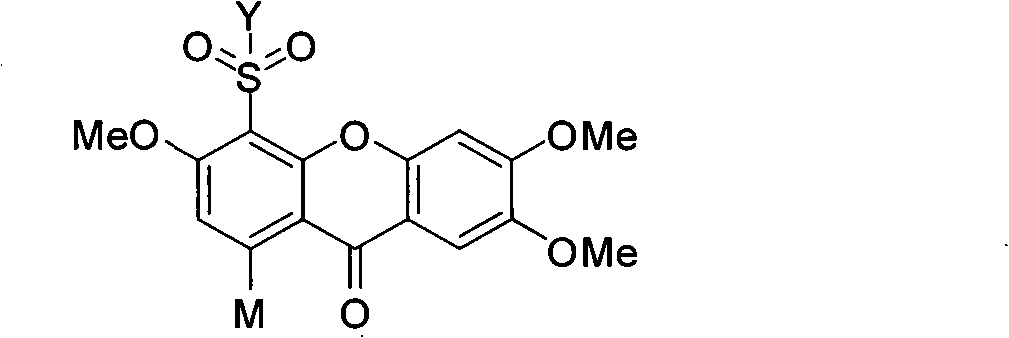
Novel sulfonyl substituted benzophenone oxide compounds, prepraring method and application thereof
A xanthone and sulfonyl technology, which is applied in the field of xanthone ACAT enzyme inhibitory active compounds, can solve problems such as affecting the normal operation of the body, unreported inhibitory activity, diseases, etc., and achieves regulation of sebaceous gland function and inhibition of sebum. overgenerated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
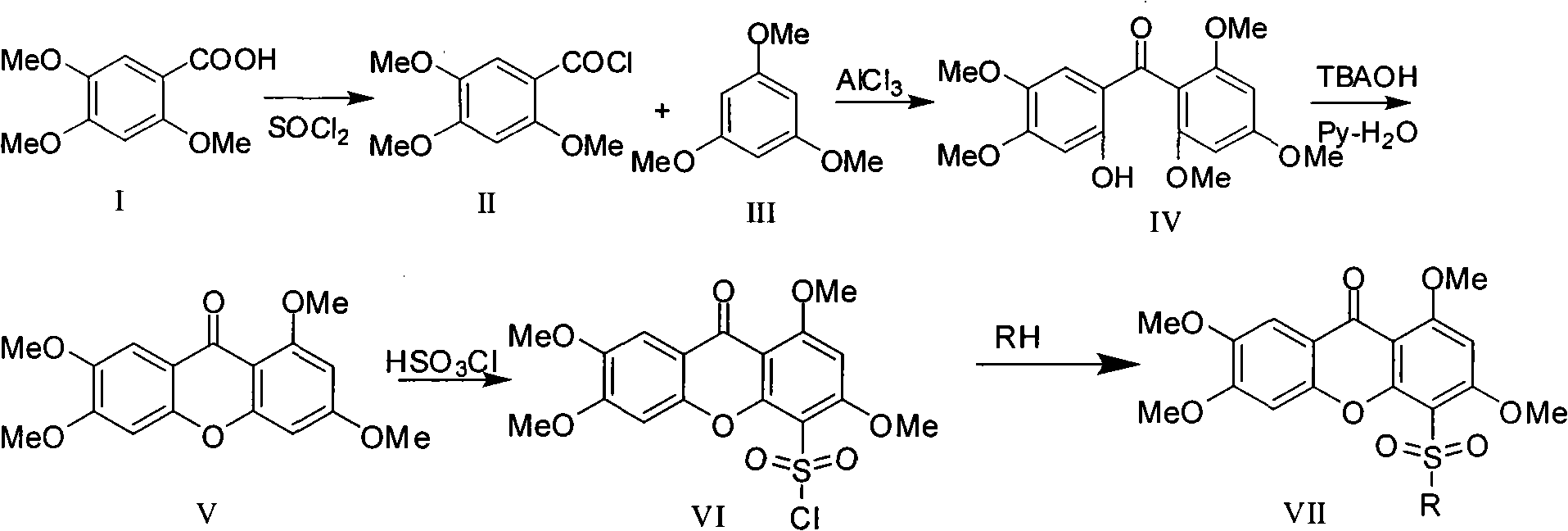
[0052] The target compound was synthesized according to the reaction route (a).
[0053] (1) Preparation of 2,4,5-trimethoxybenzoyl chloride (II)
[0054] Put 250g (1.179mol) of 2,4,5-trimethoxybenzoic acid in a 1000°C three-neck flask, stir at room temperature, slowly add 300ml of thionyl chloride dropwise, and slowly heat to reflux after the dropwise addition, continue React for 4 to 5 hours. After completion of the reaction, 150 ml of toluene was added to the reaction, and the azeotrope of thionyl chloride and toluene was distilled. When no liquid was evaporated, a white-green solid was produced upon cooling, and the crude product was dried under reduced pressure. Recrystallized from methanol to obtain 237g of 2,4,5-trimethoxybenzoyl chloride, yield 87.45%, melting point: 133-138°C.
[0055] (2) Preparation of (2-hydroxyl-4,5-dimethoxyphenyl)-(2,4,6-trimethoxyphenyl)-methanone (IV)
[0056] Put 25g (0.11mol) of 2,4,5-trimethoxybenzoyl chloride and 20g (0.12mmol) of 1,3,...
Embodiment 2
[0066] Synthesize target compound according to reaction synthetic route (b)
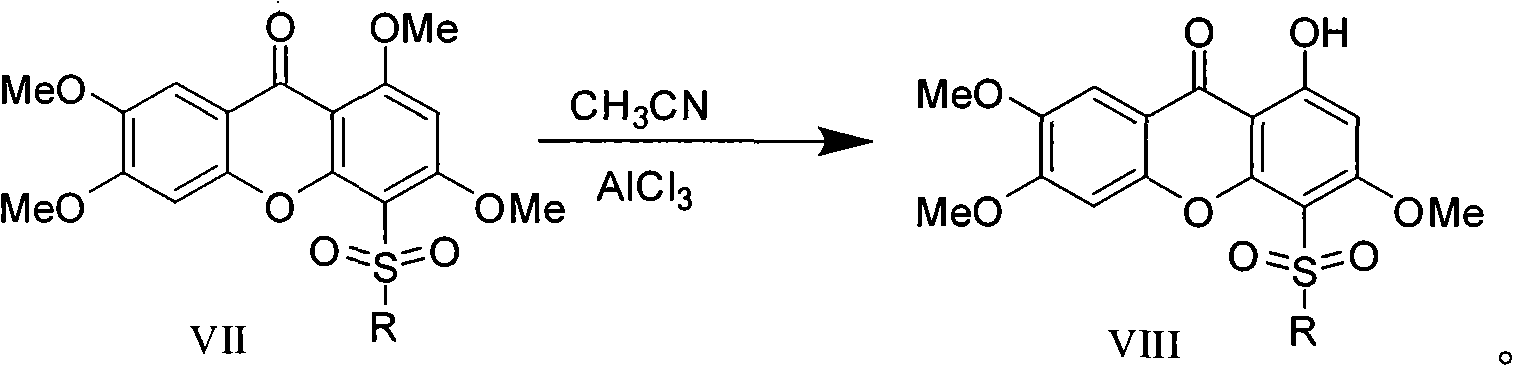
[0067] (6) Preparation of N-cyclopropylamine-1-hydroxyl-3,6,7-trimethoxy-4-sulfonamide-xanthone
[0068] Put N-cyclopropylamine-1,3,6,7-tetramethoxy-4-sulfonamide-xanthone 0.218g (0.0005mol) in a 100ml dry three-necked bottle equipped with a thermometer and a stirrer, Add 20ml of acetocyanide, heat the outside to 50°C with an oil bath, slowly add 2g of aluminum trichloride (0.0015mol) under stirring, and continue stirring for 5 hours after the addition is complete. After the reaction was completed, the reaction night was slowly poured into 100ml of 2% hydrochloric acid solution. The solution was extracted four times with 400ml ethyl acetate, and the ethyl acetate extracts were combined and dried over anhydrous sodium sulfate. Ethyl acetate was evaporated to give a crude yellow solid. The crude product was subjected to column chromatography (petroleum ether: ethyl acetate 3:1), and finally N-cyclop...
Embodiment 3
[0069] Example 3 Preparation of 4-(2S,3R)-2,6-dimethylmorphinesulfonyl-1,3,6,7-tetramethoxy-xanthone
[0070] Put 0.55g (0.0013mol) of 1,3,6,7-tetramethoxy-4-sulfonyl chloride-xanthone into 100ml dry eggplant, add 20ml 1,4-dioxane and stir , fully suspend the raw material in 1,4-dioxane, slowly add 100ul (2S,3R)-2,6-dimethylmorphine dropwise at room temperature, and react for 1 hour. After the reaction was completed, the reaction solution was poured into water, extracted with 250ml of dichloromethane four times, the dichloromethane extracts were combined, dried over anhydrous sodium sulfate, and the dichloromethane was distilled off to obtain a yellow solid crude product. The crude product was washed with a small amount of acetone, and there was a white insoluble solid, which was filtered to obtain 4-(2S,3R)-2,6-dimethylmorphinesulfonyl-1,3,6,7-tetramethoxy-xanthene Ketone 0.57g, yield 89.1% Melting point: >250°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com