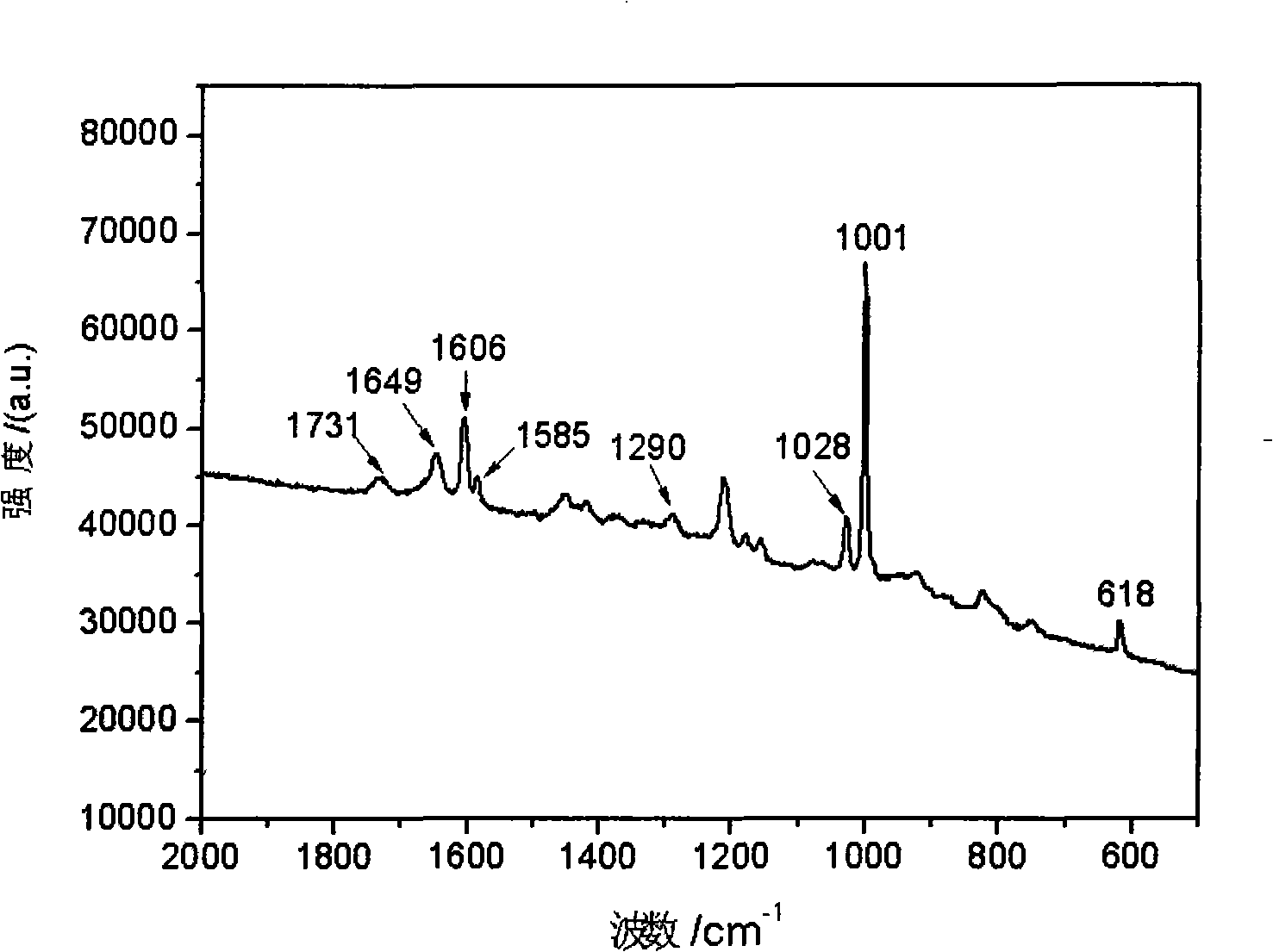
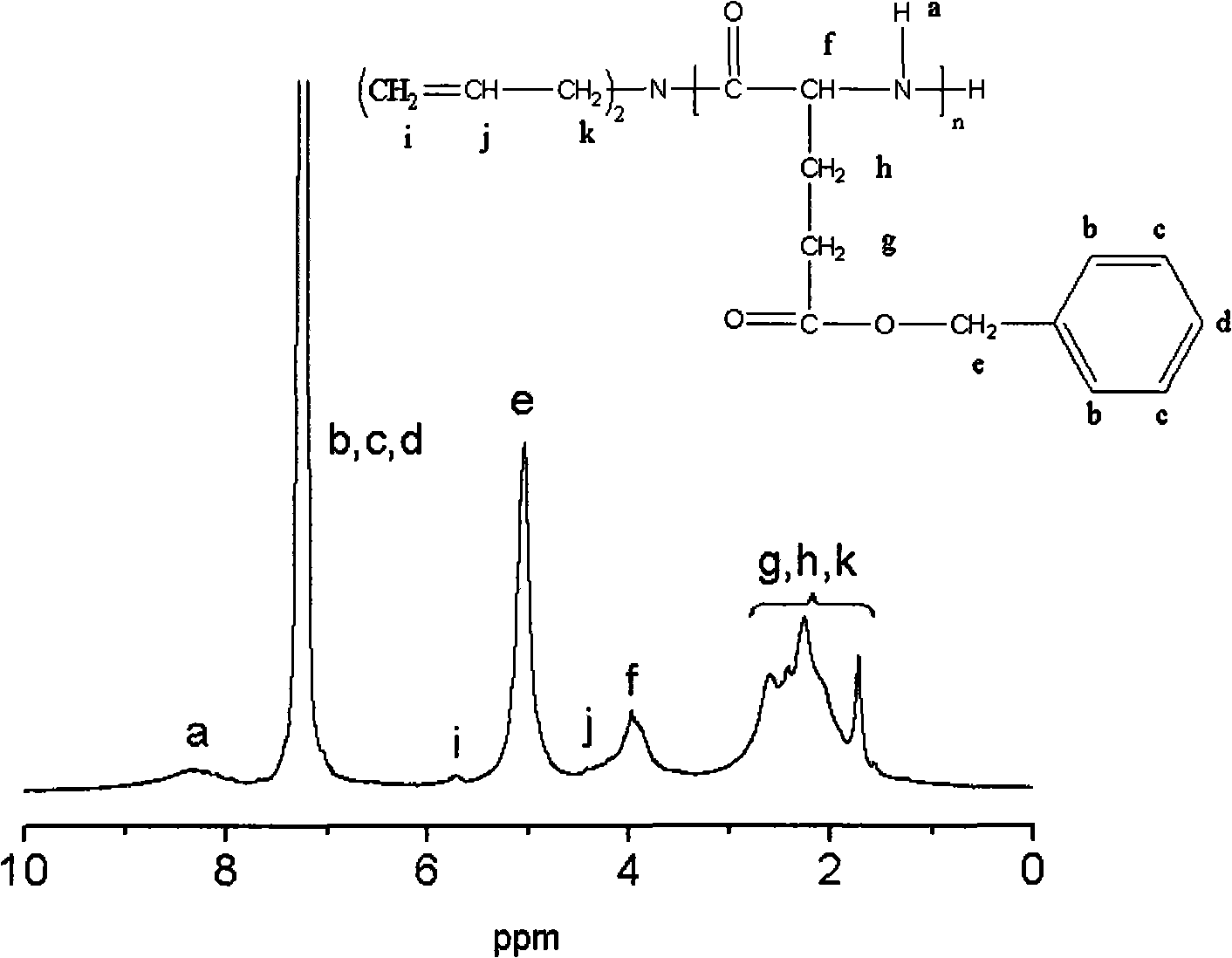
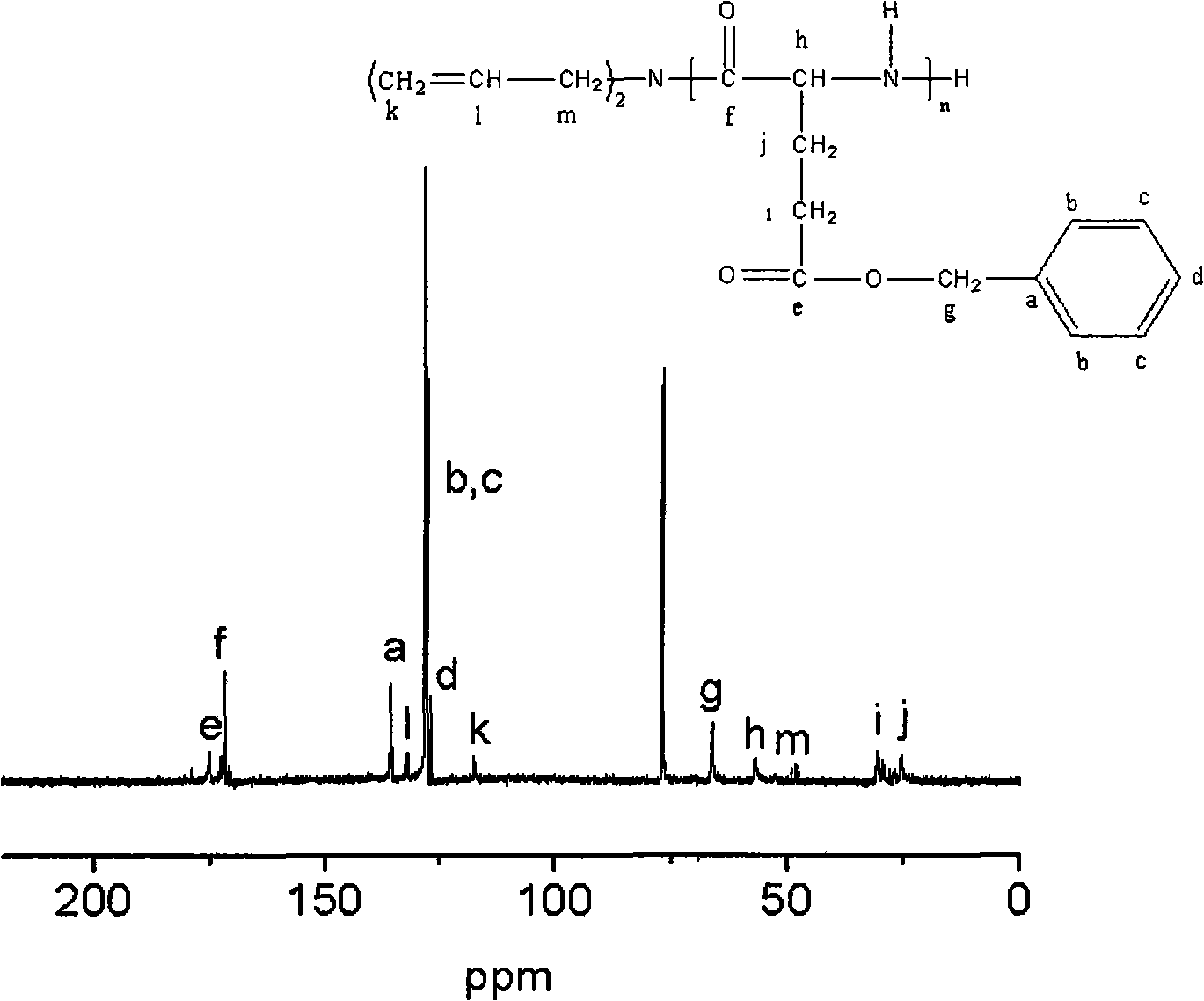
Poly benzyl glutamate-containing degradable composite material and preparation thereof
A technology of poly(benzyl glutamic acid) and composite materials, applied in the field of biomedical composite materials, can solve the problem of not containing functional groups, and achieve the effects of widening applications, shortening technical routes, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] a. Mix 10 grams of L-glutamic acid, 40 mL of benzyl alcohol and 14.5 mL of hydrobromic acid solution (30% by weight), and heat to 60° C.; after the reaction solution becomes clear, cool down to room temperature, and mix The solution was poured into 500 mL of pyridine and ethanol mixture with a volume ratio of 1:5 to obtain a white precipitate, which was placed overnight at 2°C; filtered, washed with ethanol and ether to obtain a crude product, which was recrystallized with ethanol and dried to obtain White crystalline product, L-glutamic acid-γ-benzyl ester;
[0030] b. Dissolve 5 grams of L-benzyl glutamate in 28 mL of tetrahydrofuran, and when the temperature rises to 40° C., add 4 grams of bis(trichloromethyl) carbonate; stop heating after the reaction mixture becomes clear, and fill with nitrogen 30 minutes; then the reaction solution was concentrated to 20 mL, poured into 100 mL of anhydrous petroleum ether to obtain flocculent crystals, the precipitate was filtere...
Embodiment 2
[0040] (1) Synthesis of γ-benzyl-L-glutamic acid-N-carboxylic acid anhydride
[0041] a. Mix 10 grams of L-glutamic acid, 60 mL of benzyl alcohol and 36 mL of hydrobromic acid solution (the percentage concentration is 45%), heat to 80 ° C; Pour into 500mL of pyridine and ethanol mixture with a volume ratio of 1:8 to obtain a white precipitate, which is placed overnight at 4°C; filter, wash with ethanol and ether respectively to obtain a crude product, recrystallize the crude product with ethanol, and dry to obtain a white crystal The product, namely L-glutamic acid-γ-benzyl ester;
[0042] b. Dissolve 5 grams of L-benzyl glutamate in 75 mL of tetrahydrofuran, and when the temperature rises to 55° C., add 7.5 grams of bis(trichloromethyl)carbonate, stop heating after the reaction mixture becomes clear, and fill with nitrogen 45 minutes; then the reaction solution was concentrated to 20 mL, poured into 120 mL of anhydrous petroleum ether to obtain flocculent crystals, filtered,...
Embodiment 3
[0046] (1) Synthesis of γ-benzyl-L-glutamic acid-N-carboxylic acid anhydride
[0047] a. 10 grams of L-glutamic acid, 50 mL of benzyl alcohol and 29 mL of hydrobromic acid solution (38% by weight) were mixed and heated to 70 ° C; after the reaction solution became clear, it was cooled to room temperature, and the mixed solution was Pour it into 400 mL of pyridine and ethanol mixture with a volume ratio of 1:6 to obtain a white precipitate, which is placed at 3°C overnight; filter, wash with ethanol and ether respectively to obtain a crude product, recrystallize the crude product with ethanol, and dry to obtain a white Crystalline product, namely L-glutamic acid-γ-benzyl ester;
[0048] b. Dissolve 5 g of benzyl L-glutamate in 56 mL of tetrahydrofuran, and when the temperature rises to 50° C., add 6 g of bis(trichloromethyl)carbonate; stop heating after the reaction mixture becomes clear, and fill with nitrogen 60 minutes; then the reaction solution was concentrated to 20 mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com