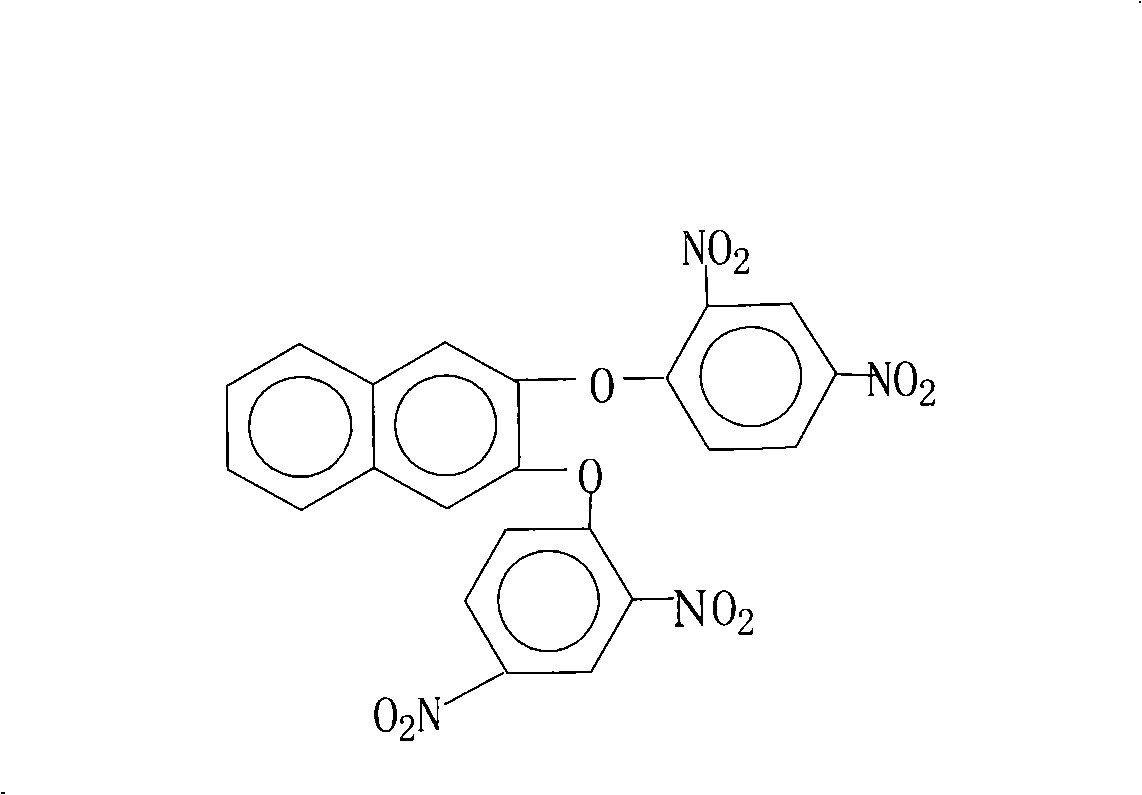
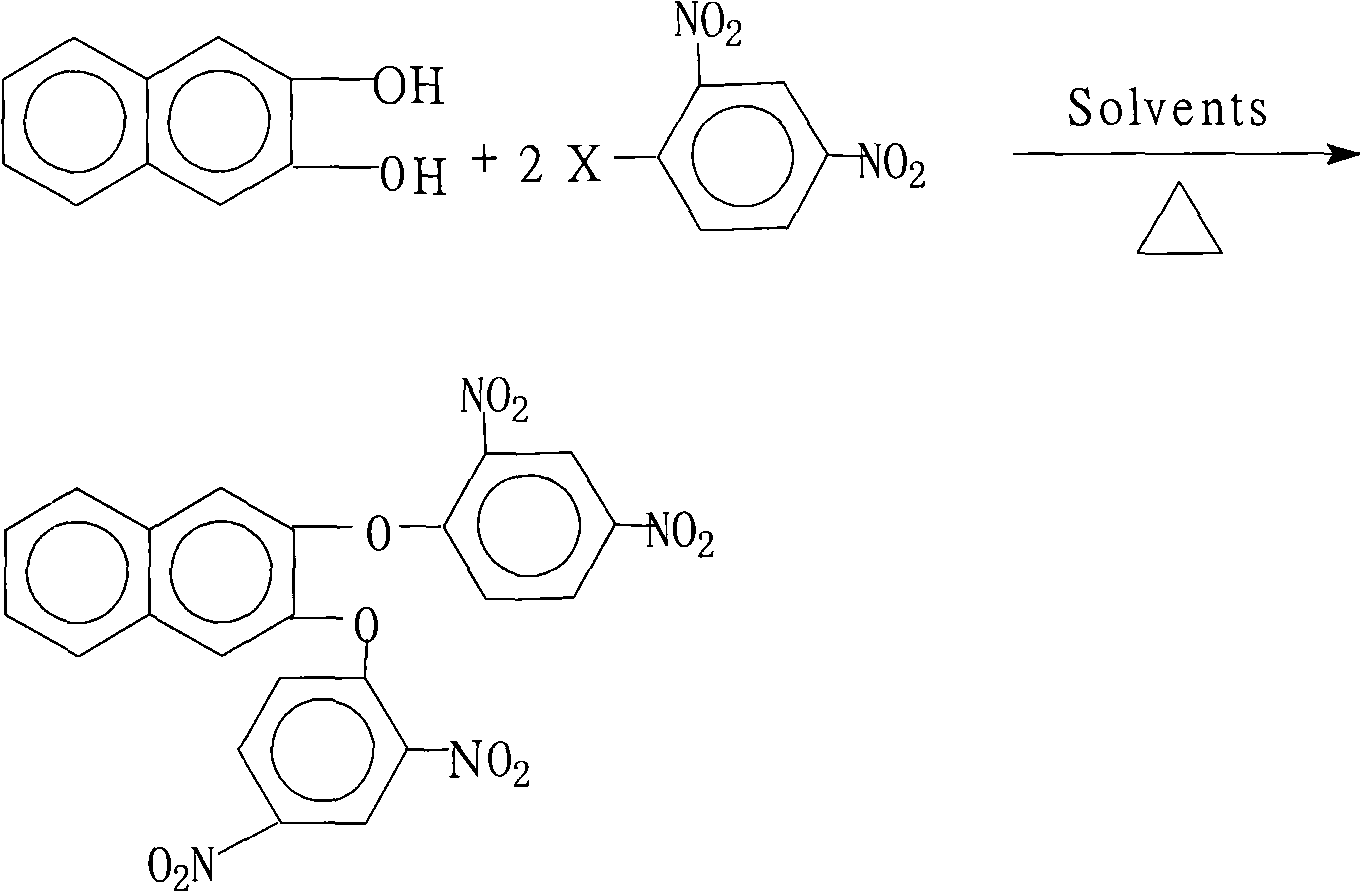
Preparation method of 2,3-di(2,4-dinitro phenoxy)naphthalin
A technology of dinitrophenoxy and dinitro, which is applied in 2 fields to achieve the effects of convenient source, simple operation, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 16.0 grams (0.10 moles) of 2,3-naphthalenediol, 44.6 grams (0.22 moles) of 2,4-dinitrochlorobenzene, 110.4 grams (0.80 moles) of potassium carbonate, 200 milliliters of N-methyl-2- Pyrrolidone, 500 milliliters of N, N-dimethylformamide and 180 milliliters of toluene were put into a reaction kettle, stirred, heated to reflux and separated from water for 18 hours, then concentrated the reaction liquid, recovered the solvent for recycling, cooled the reactant system, and added water , separate out the solid product, wash 2~3 times with hot water, dry, obtain 44.3 grams of 2,3-bis(2,4-dinitrophenoxy)naphthalene crystal product, the purity is 99.1%, according to the actual obtained 2 , 3-bis(2,4-dinitrophenoxy)naphthalene amount and theoretical amount (49.2 grams), calculate the yield of 2,3-bis(2,4-dinitrophenoxy)naphthalene was 90.1%.
Embodiment 2
[0028] 16.0 grams (0.10 moles) of 2,3-naphthalenediol, 54.4 grams (0.22 moles) of 2,4-dinitrobromobenzene, 55.2 grams (0.40 moles) of potassium carbonate, 150 milliliters of N-methyl-2- Pyrrolidone, 150 milliliters of N, N-dimethylacetamide, 100 milliliters of benzene and 15 milliliters of xylene were put into the reaction kettle, stirred, heated to reflux and separated from water for 6 hours, concentrated the reaction liquid, recovered the solvent for recycling, cooled The reactant system was added with water to precipitate a solid product, washed 2 to 3 times with hot water, and dried to obtain 38.8 grams of 2,3-bis(2,4-dinitrophenoxy)naphthalene crystal product with a purity of 99.5%. According to the actual amount of 2,3-bis(2,4-dinitrophenoxy)naphthalene and the theoretical amount (49.2 grams), 2,3-bis(2,4-dinitrophenoxy) was calculated The yield of naphthalene was 78.9%.
Embodiment 3
[0030] 16.0 grams (0.10 moles) of 2,3-naphthalenediol, 40.5 grams (0.20 moles) of 2,4-dinitrochlorobenzene, 10.6 grams (0.10 moles) of sodium carbonate, 180 milliliters of N-methyl-2- Pyrrolidone, 80 milliliters of benzene and 30 milliliters of dichlorobenzene were put into a reaction kettle, stirred, heated to reflux and separated from water for 16 hours, concentrated the reaction solution, recovered the solvent for recycling, cooled the reactant system, added water, and precipitated a solid product. Wash 2~3 times with hot water, dry, obtain 34.5 grams of 2,3-bis(2,4-dinitrophenoxy)naphthalene crystal product, the purity is 99.1%, according to the actual obtained 2,3-bis( The amount of 2,4-dinitrophenoxy)naphthalene and the theoretical yield (49.2 g), the calculated yield of 2,3-bis(2,4-dinitrophenoxy)naphthalene was 70.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com