Method of preparing ultra-fine danazol powder
A technology of danazol and azole powder, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, powder delivery, etc., which can solve the problem of affecting the quality of danazol powder and the narrow particle size distribution of danazol , uneven mixing and other problems, to achieve the effect of easy packaging, shortened reaction time, and uniform particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
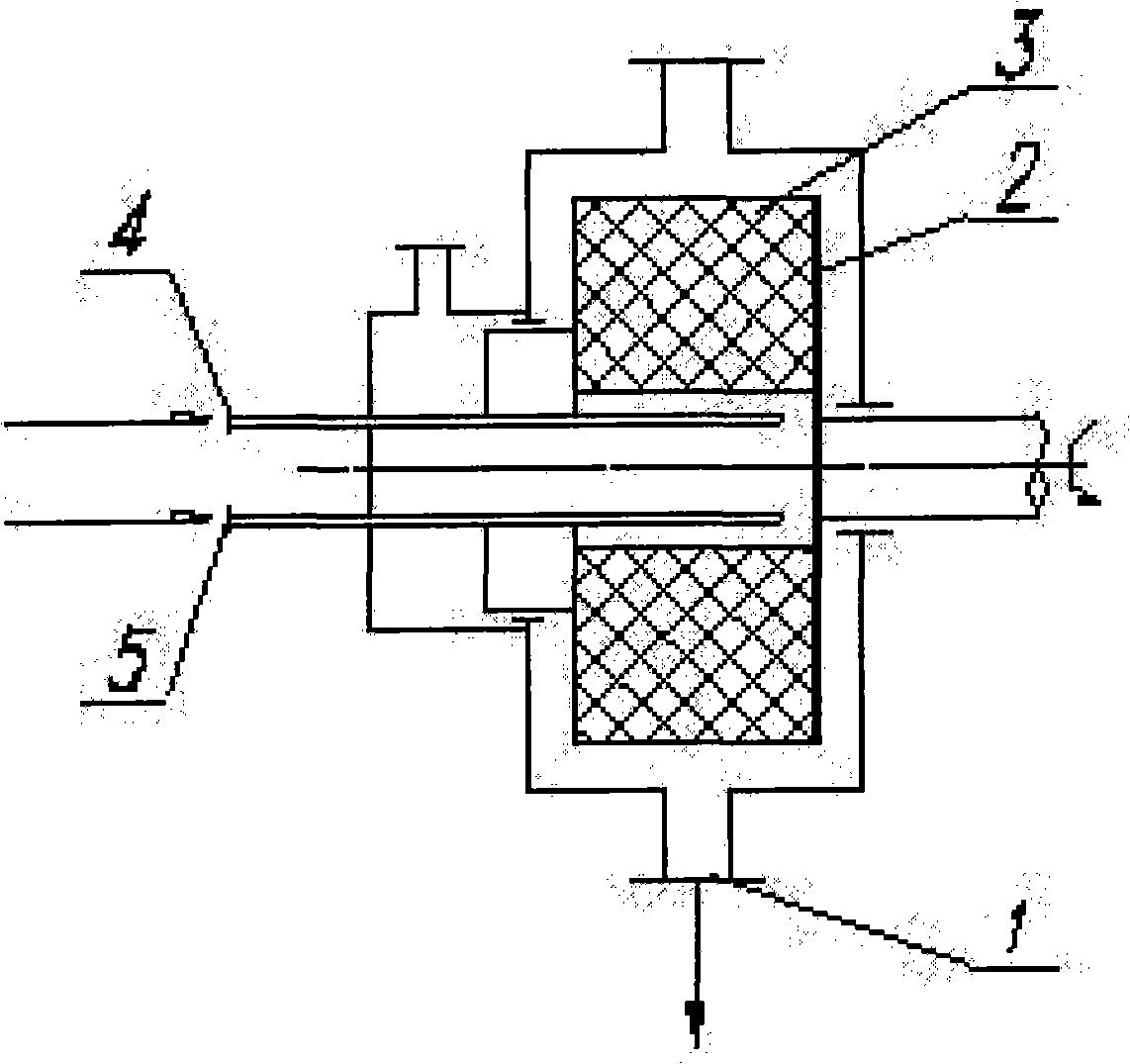
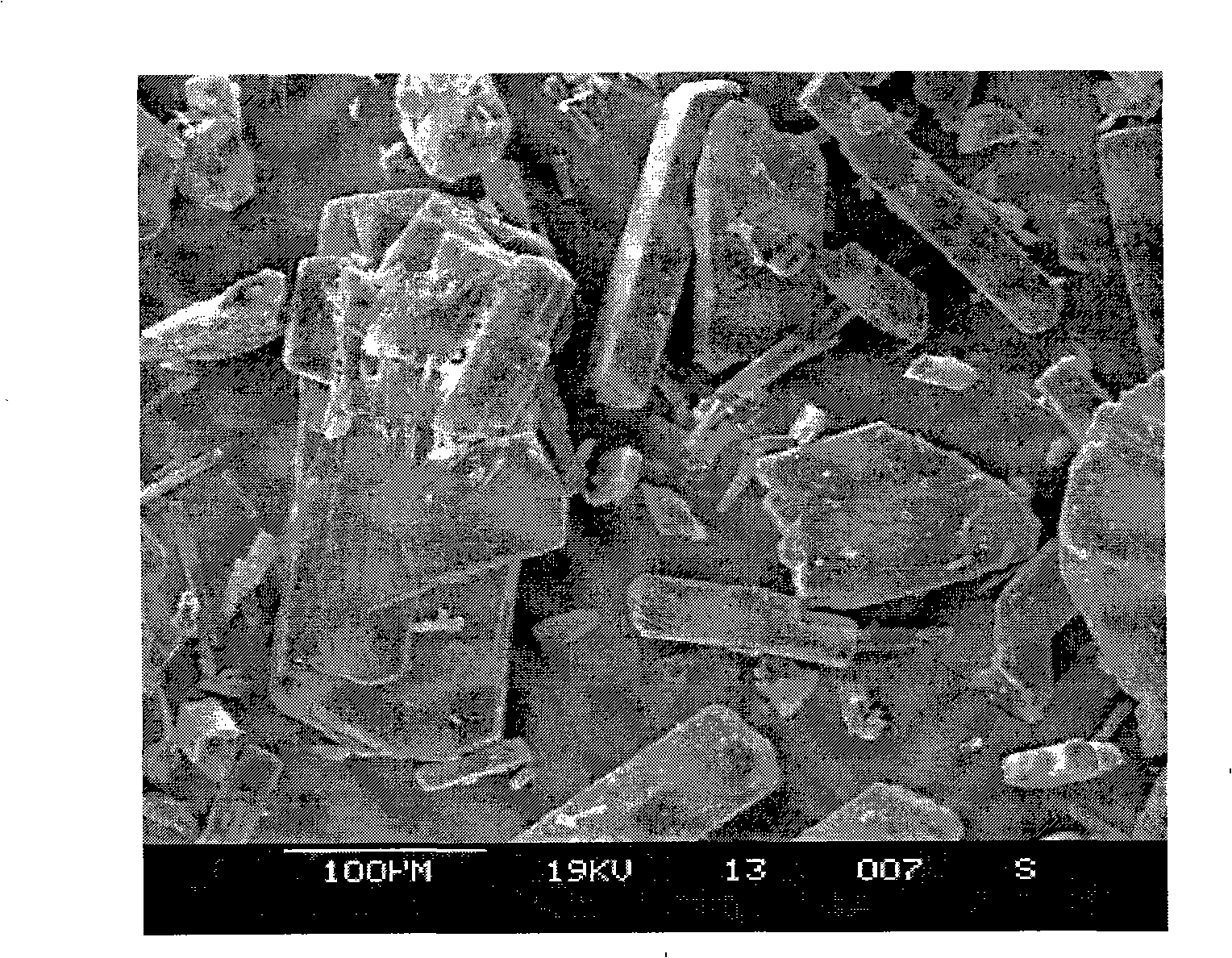
[0041] Take by weighing 20g of danazol crude drug, dissolve and prepare 1000ml of danazol solution with ethanol, place in storage tank, get deionized water 40L (liter) and place another storage tank as anti-solvent, two storage tanks pass through respectively The pipeline is connected with the solution inlet 4 and the anti-solvent inlet 5 of the high-gravity rotating packed bed reactor. At room temperature, the two liquids are pumped in respectively from the solution inlet 4 and the anti-solvent inlet 5, and sprayed into the filler 3 through the liquid distributor. The temperature in the rotating bed is controlled at 4°C. Precipitation crystallizes to generate white danazol precipitate, which flows out from the outlet 1 of the rotary bed, is filtered, washed, and dried to obtain danazol granules. During operation, the flow ratio of danazol solution / anti-solvent was 1:40, and the rotational speed of the rotating bed was 3000 rpm. from image 3 It can be seen from the scanning...
Embodiment 2
[0043]Weigh 80 g of danazol raw material, dissolve and prepare 1000 ml of danazol solution with acetone, take 20 L of deionized water as anti-solvent, control the temperature in the rotating bed at 4 ° C, and the ratio of danazol solution to anti-solvent is 1: 20, Prepared ultrafine amorphous danazol particles from Figure 4 It can be seen from the scanning electron microscope photos that the average particle size is about 1 μm.
Embodiment 3
[0045] Weigh 400g of danazol bulk drug, prepare 2 liters of danazol solution with dichloromethane, take 10L of cyclohexane as anti-solvent, control the temperature in the rotating bed at 30°C, and the flow ratio of danazol solution / anti-solvent is 1:5, the rotating bed rotating speed is 1000rpm, all the other are with embodiment 1, make superfine amorphous danazol particle, from Figure 5 It can be seen from the scanning electron microscope photos shown that the average particle size is about 20 μm in length and about 4 μm in width, and the shape is uniform needle-shaped particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com