System for inducing nerve stem cell differentiation and inducing method thereof
A technology of neural stem cells and cells, which is applied in the fields of biotechnology and cell biology, and can solve problems such as the limitation of the developmental potential of neural stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P19
[0166] Example 1P19 cells are equivalent to epiblast cells in early embryos
[0167] It has been reported that P19 cells are similar to epiblast cells in early embryos in terms of developmental period. In order to further confirm this statement, the inventors used RT-PCR, immunostaining and in situ hybridization experiments to detect the expression of molecular markers of inner cell mass and epiblast in ES cells and P19 cells.
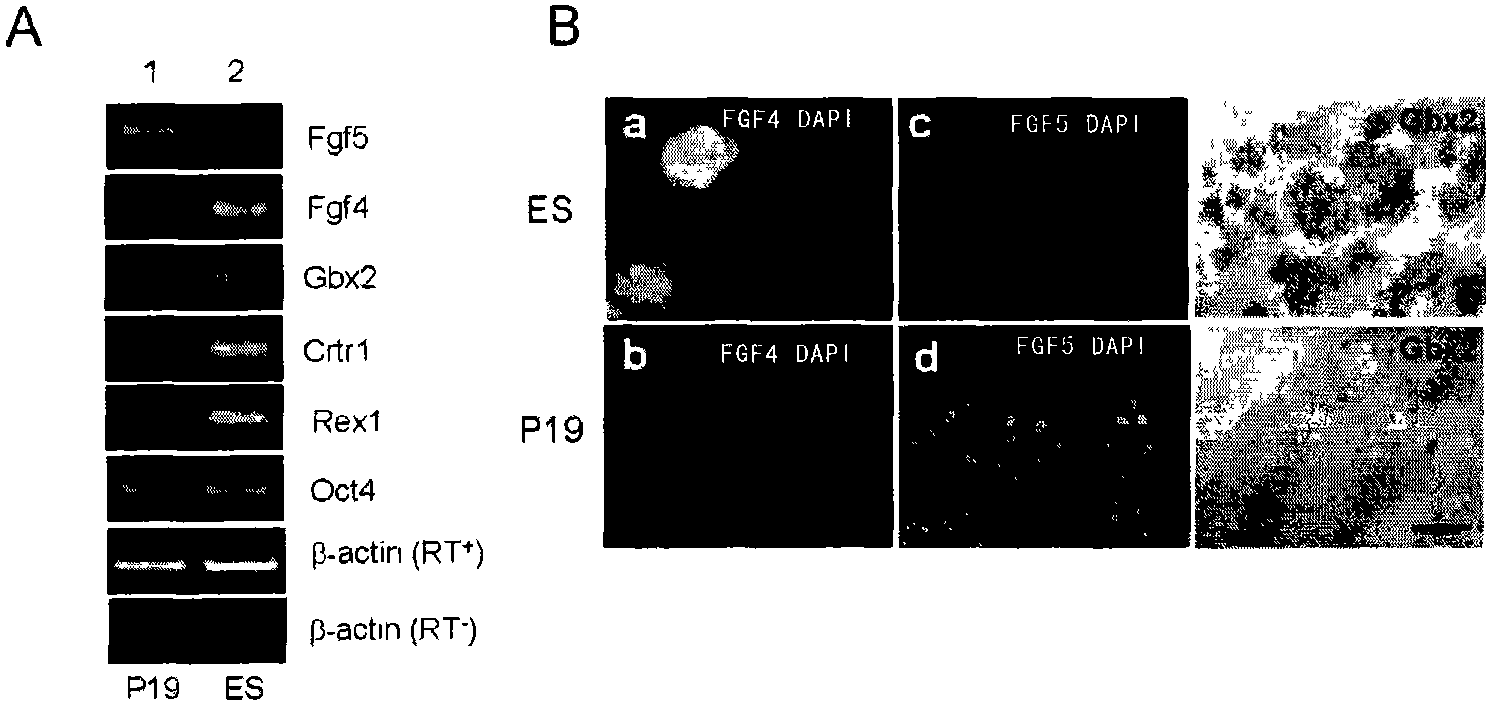
[0168] RT-PCR results see figure 1 a. The results showed that P19 cells expressed epiblast molecular marker FGF5, but did not express inner cell mass (Inner cell mass) molecular markers FGF4, Gbx2, Rex1, CRTR-1; on the contrary, ES expressed inner cell mass The molecular markers FGF4, Gbx2, Rex1 and CRTR-1 were expressed, but the molecular marker FGF5 of the epiblast was not expressed.
[0169] The results of immunostaining for FGF4 and FGF5 and in situ hybridization for Gbx2 are consistent with the results of RT-PCR, see figure 1 b.
[0170] Alth...
Embodiment 2N2B27
[0171] Example 2 N2B27 can induce P19 cells to differentiate into a high proportion of neural stem cells
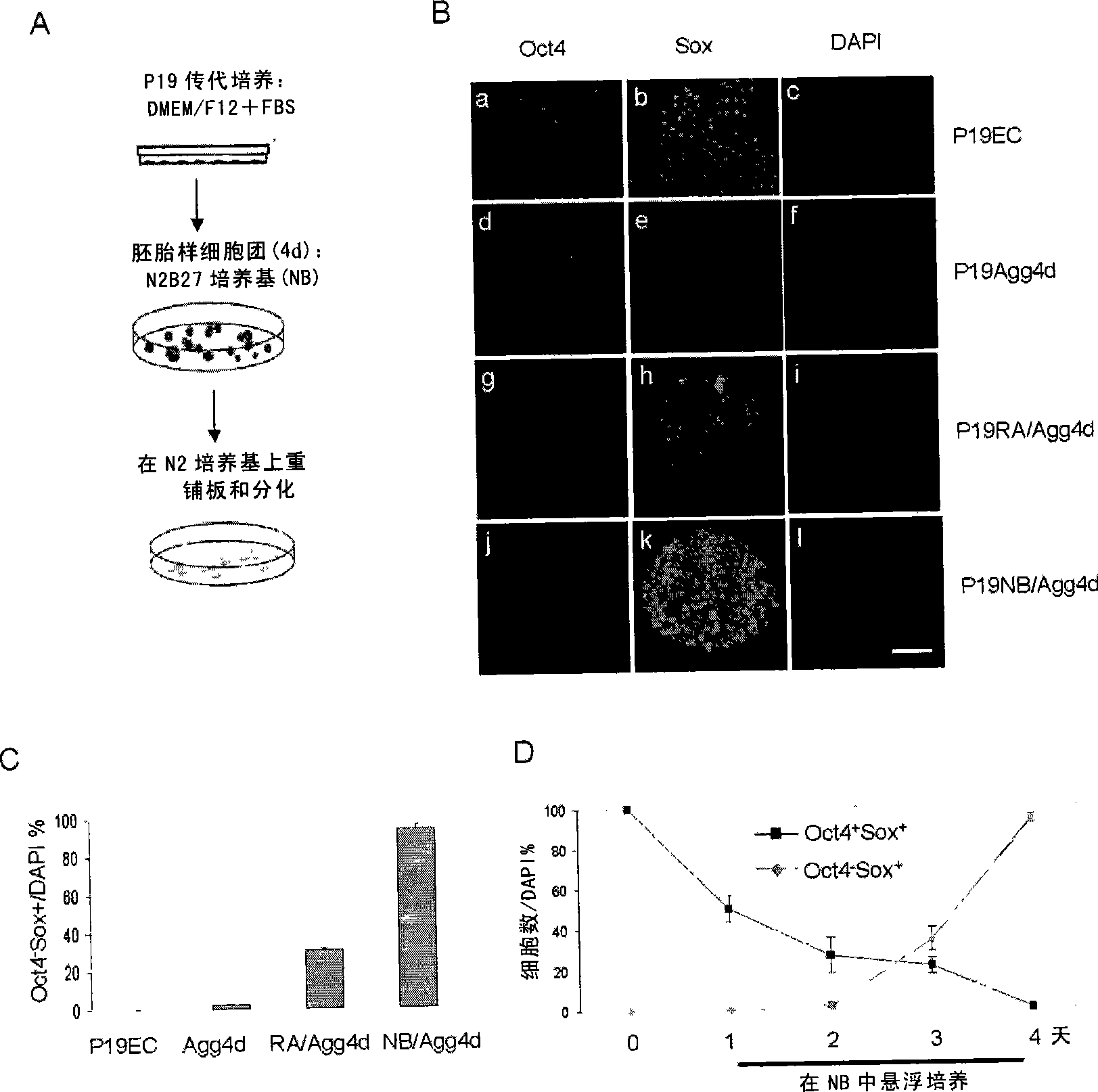
[0172] P19 cells were suspended and cultured in N2B27 serum-free medium for 4 days (P19NB / Agg4d). After 4 days, a large number of cell clusters could be seen suspended in the culture medium. The cell clusters were carefully collected by natural sedimentation, fixed, and frozen sectioned to investigate the expression of stem cell-specific molecular marker Oct4 and neural stem cell-specific molecular marker Sox in P19 cell clusters induced by N2B27 for 4 days .
[0173] It was found that uninduced P19 cells were pluripotent and all cells were Oct4 + sox + , cells with stem cell properties. After 4 days of suspension culture in serum-containing medium, the cell mass without RA treatment (P19Agg4d) no longer expressed Sox, but continued to express the molecular marker Oct4 of pluripotent cells; induced by RA (P19RA / Agg4d) or N2B27 (P19NB / Agg4d) The 4-day-old cell pellet n...
Embodiment 3
[0178] The neural induction of embodiment 3N2B27 is not accompanied by the induction of mesoderm and endoderm
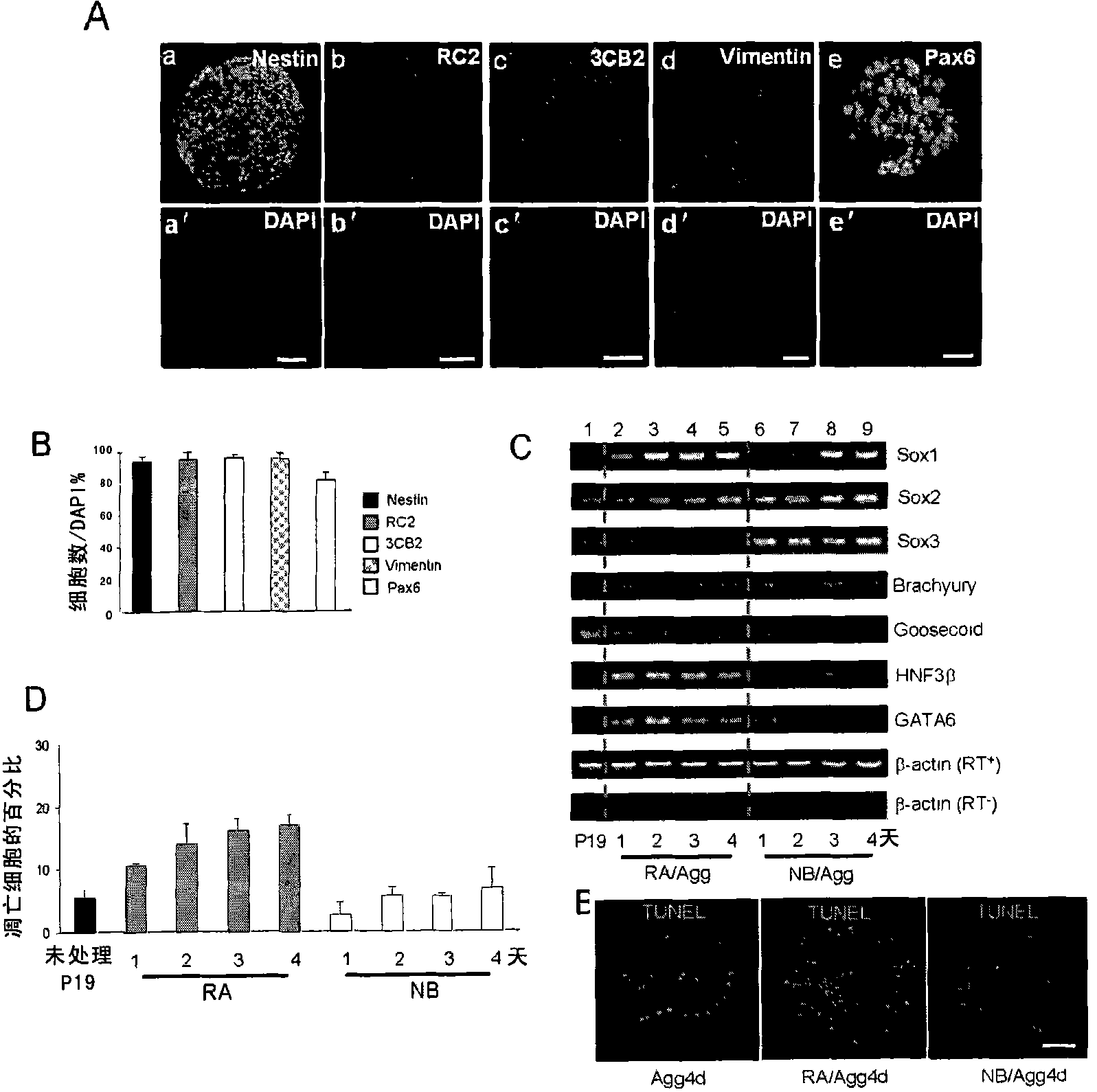
[0179] Neural induction in serum-containing medium is often accompanied by the emergence of mesoderm and endoderm cells. To test whether this is also the case for neural induction in N2B27 medium, the inventors detected the expression of molecular markers of the three germ layers by RT-PCR.
[0180] It was found that the expression of Sox1, 2, 3, the molecular markers of neuroectoderm, was up-regulated after N2B27 induction (NB / Agg). It is especially noteworthy that the expression of Sox1 was greatly up-regulated on the third day of induction, which is consistent with the expression of neural stem cells induced by N2B27. Consistent results began to appear on Day 3. Mesoderm molecular markers Brachyury and Goosecoid were only expressed at low levels during N2B27 induction, while endoderm molecular markers HNF3β and GATA6 were almost undetectable; these molecular mark...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com