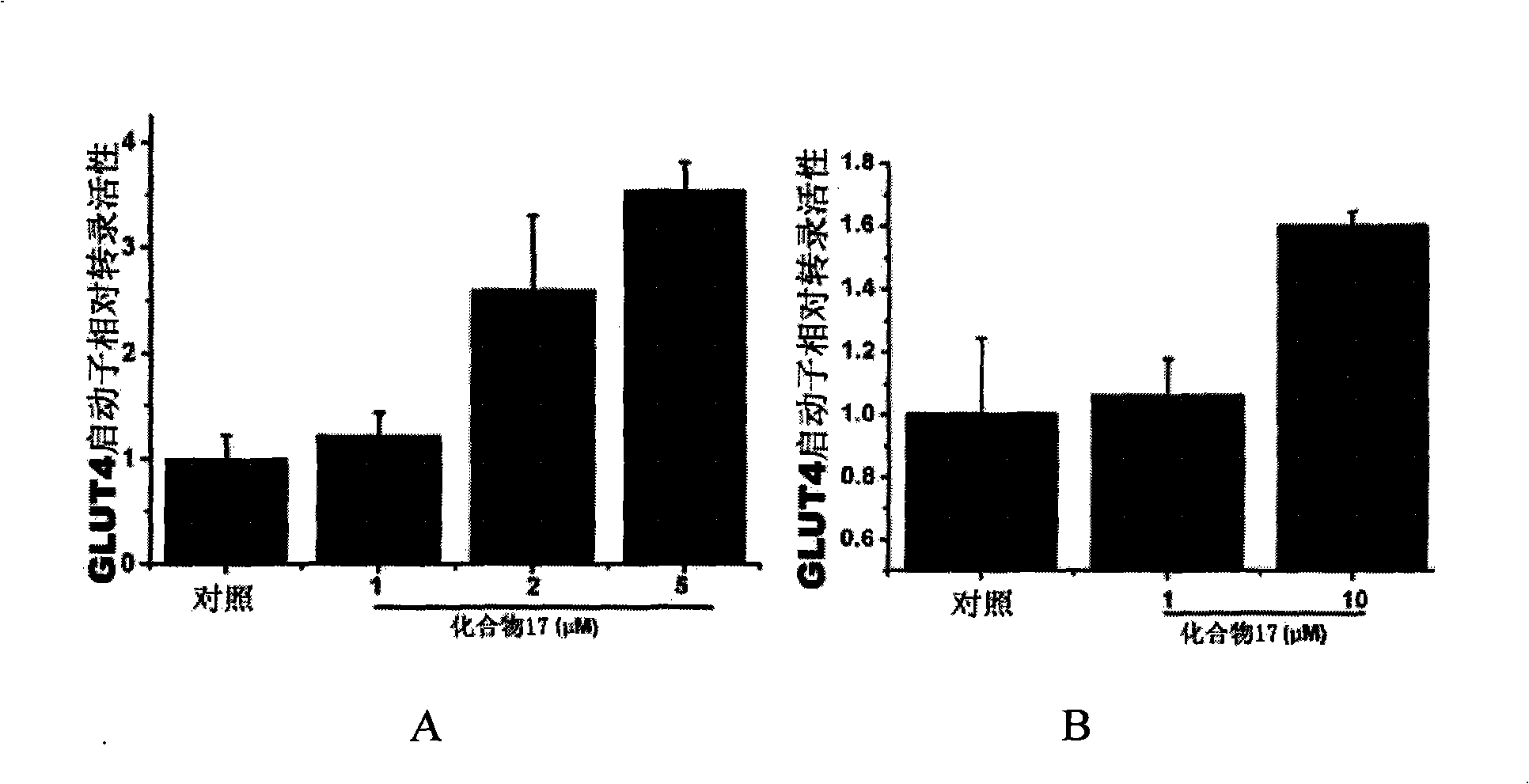
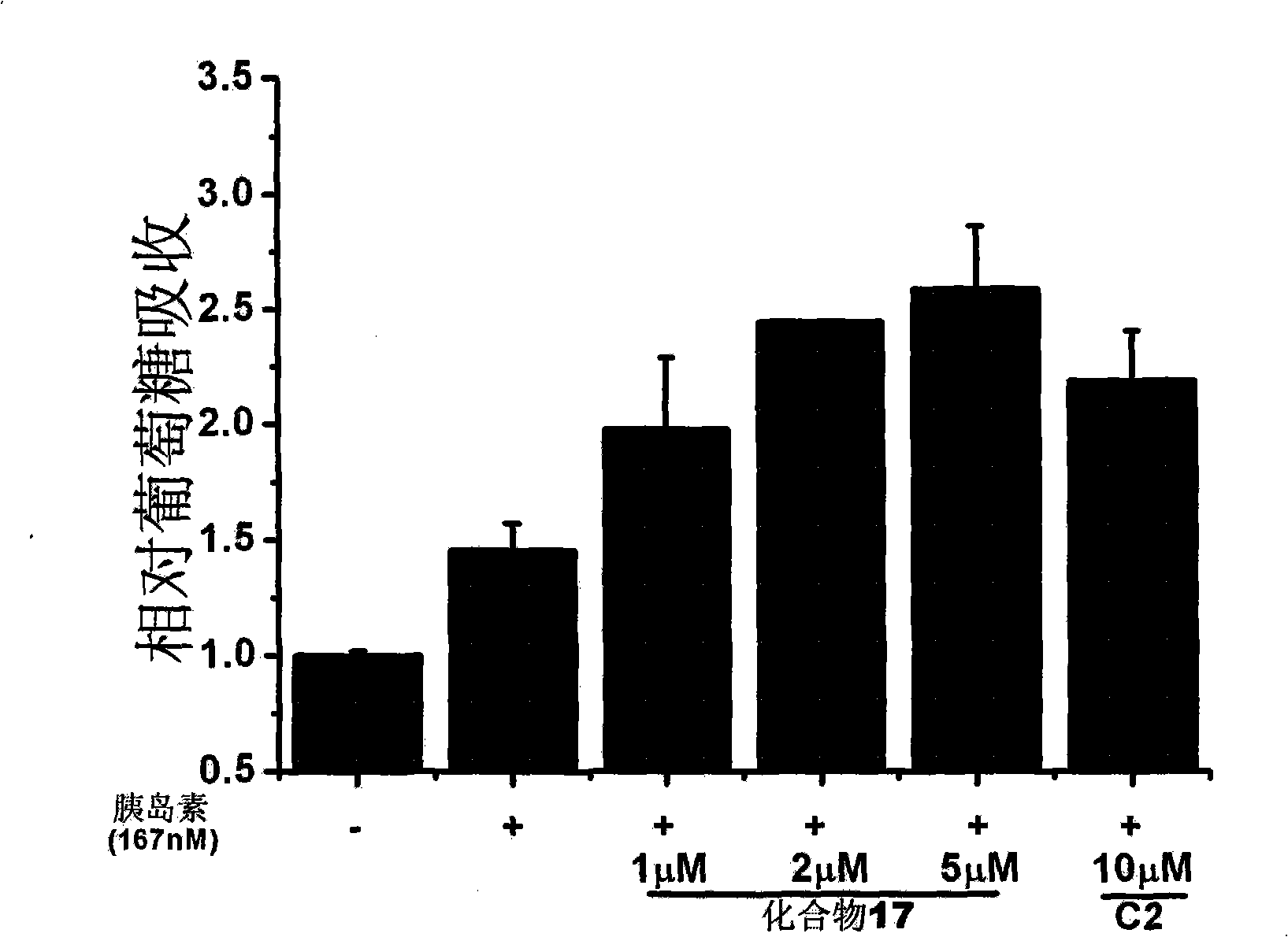
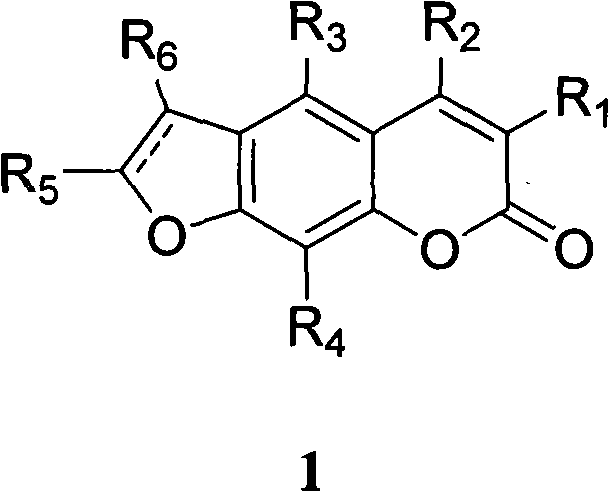
Linear furocoumarin derivates, preparation method and application thereof, pharmaceutical compositions containing the derivates
A furanocoumarin and derivative technology, which can be used in drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as weakening and blocking of insulin signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0071] Preparation Example 1 Preparation of Compound 2
[0072] (1) Preparation of 6-hydroxy-7-dimethoxycoumarin (2a)
[0073] For convenience, M in the following structural formula represents Me, that is, methyl.
[0074]
[0075] Methoxychloromethane (0.443mL, 5.84mmol) was added to aescin (520.5mg, 2.92mmol) and potassium carbonate (605mg, 4.38mmol) in N,N-dimethylformamide (14.6mL) solution, and After stirring for 13 hours at -20°C, the reaction solution was diluted with 10 mL of water, and then 1 equivalent of hydrochloric acid was added dropwise at 0°C until the solution changed from yellow to colorless. The aqueous layer was extracted with ethyl acetate (2×10 mL), the organic layers were combined, washed with saturated brine (10 mL), dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 9: 2 elution) to obtain compound 2a as a yellow solid.
[0...
preparation Embodiment 2
[0096] Preparation Example 2 Preparation of Compound 3
[0097] (1) Preparation of 4-methoxymethyl-7-hydroxycoumarin (3a)
[0098]
[0099] To a mixed solution of resorcinol (5.0g, 30mmol) and concentrated sulfuric acid (100mL) was added dropwise methyl-4-methoxyethyl acetoacetate (4.4g, 30mmol), and stirred at 0°C overnight, After the reaction was complete, the reaction solution was poured into ice water, stirred at room temperature for 1 hour, extracted with ethyl acetate (2×100 mL), combined the organic layers, washed with saturated brine (100 mL), dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The residue was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain compound 3a (2.53 g, 38%).
[0100] (2) Preparation of 4-methoxymethyl-4'-methylpsoralen (3)
[0101]
[0102] (a) To an anhydrous acetone solution (100 mL) of 4-methoxymethyl-7-hydroxycoumarin (20.0 mmol) was added 10 g of potassium c...
preparation Embodiment 3
[0106] Preparation Example 3 Preparation of Compound 4
[0107]
[0108] According to the method of Preparation Example 2, using chloroacetophenone instead of monochloroacetone, compound 4 was prepared with a yield of 63%.
[0109] 1 H NMR(300MHz, CDCl 3 )δ: 7.92 (1H, s, 5-H), 7.81 (1H, s, 5'-H), 7.63-7.41 (6H, m, 4'-C 6 H 5 and 8-H), 6.53(1H, s, 3-H), 4.69(2H, s, 4-CH 2 -CH 3 ), 3.53(3H, s, 4-CH 2 CH 3 );
[0110] 13 C NMR(75MHz, CDCl 3 )δ: 130.9(C-1”), 129.2(C-3” and C-5”), 128.0(C-4”), 127.4(C-4” and C-6”), 123.9(C-4 '), 122.2(C-6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com