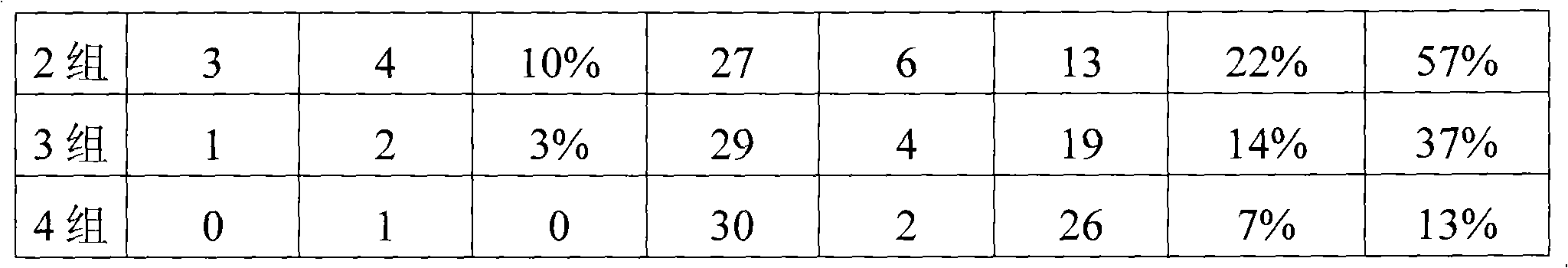Intestine toxicity syndrome vaccine oral liquid synergistic agent for livestock, poultry and aquatic product, and preparation method thereof
A technology for enterotoxic syndrome and aquatic animals, applied in the direction of medical raw materials derived from reptiles, antiviral agents, drug combinations, etc., can solve problems such as reduced electrolyte absorption, intestinal absorption disorders, and limited preventive effects, and achieve absorption rate High, wide distribution, convenient administration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
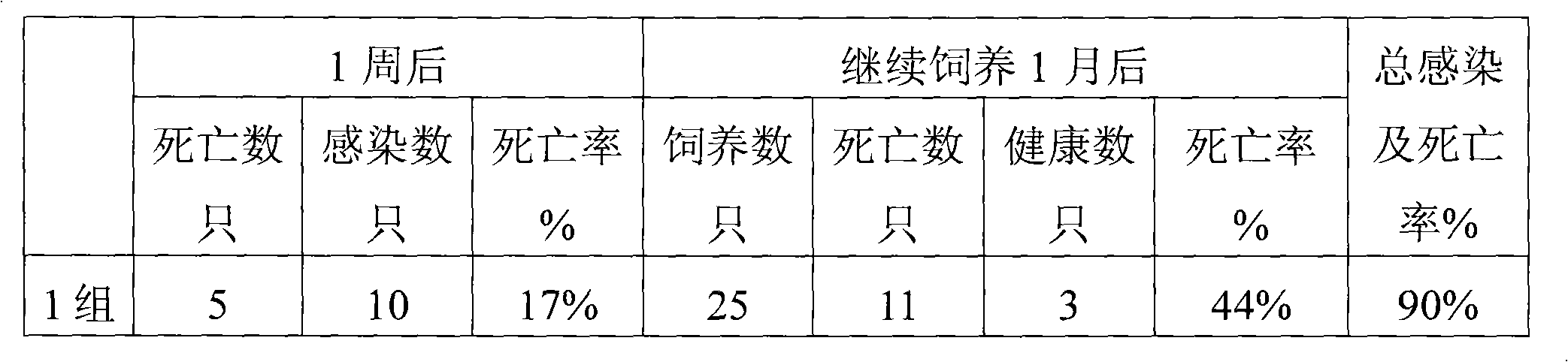
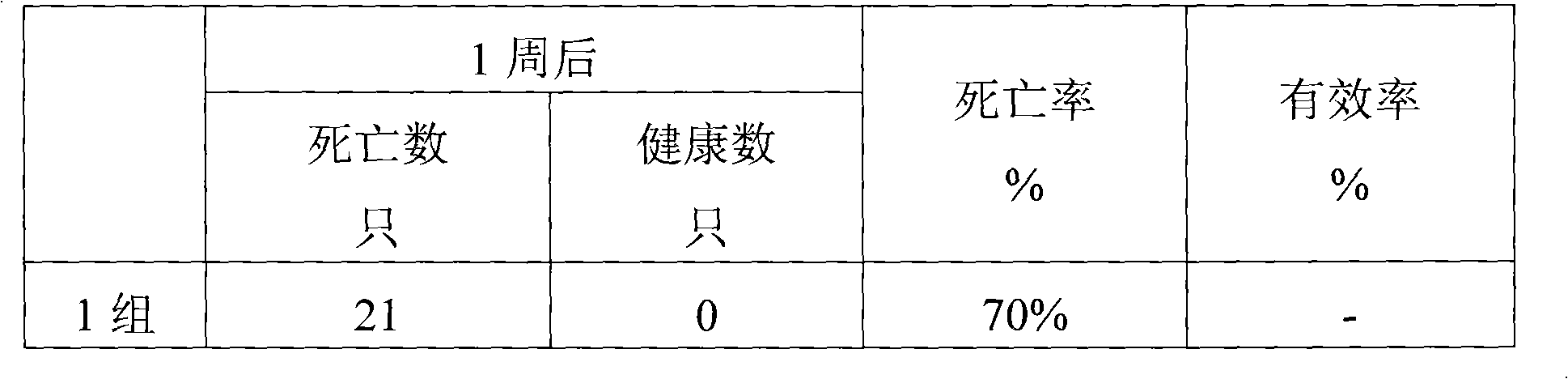
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of Oral Liquid Synergist 1 of the present invention
[0020] (1) Weigh 10 grams of Atractylodes macrocephala, 15 grams of Codonopsis, 10 grams of Artemisia annua, 10 grams of myrobalan, 20 grams of pomegranate peel, 15 grams of poplar flower, 10 grams of Alisma, 2 grams of snake gall, 10 grams of ox gall, 40 grams of aloe, Rock sugar 25 grams.
[0021] (2) Decoct the raw material twice, add 8 times the amount of water for the first time, decoct for 2 hours, add 6 times the amount of water for the second time, decoct for 1 hour, combine the two decoctions, filter to obtain the filtrate, and concentrate to specific gravity 1.35 extract, add 85-95% edible glacial acetic acid for extraction, recover glacial acetic acid and concentrate, dry at 45-50°C to obtain 15.07 grams of dry extract;
[0022] (3) Grinding the dry extract into powder, adding 18 grams of neomycin, 10 grams of oxytetracycline hydrochloride and 8 grams of sulfamethoxazole sodium, ...
Embodiment 2
[0024] Embodiment two: preparation of oral liquid synergist 2 of the present invention
[0025] (1) Weigh 20 grams of Atractylodes macrocephala, 25 grams of Codonopsis pilosula, 20 grams of Artemisia annua, 25 grams of myrobalan, 25 grams of pomegranate peel, 20 grams of poplar flower, 20 grams of Alisma, 5 grams of snake gall, 20 grams of ox gall, 60 grams of aloe, Rock sugar 40 grams.
[0026] (2) Decoct the raw material twice, add 8 times the amount of water for the first time, decoct for 2 hours, add 6 times the amount of water for the second time, decoct for 1 hour, combine the two decoctions, filter to obtain the filtrate, and concentrate to specific gravity 1.35 extract, add 85-95% edible glacial acetic acid for extraction, recover glacial acetic acid and concentrate, dry at 45-50°C to obtain 24.37 grams of dry extract;
[0027] (3) Grinding the dry extract into powder, adding 25 grams of neomycin, 16 grams of oxytetracycline hydrochloride and 10 grams of sulfamethoxaz...
Embodiment 3
[0029] Embodiment three: preparation of oral liquid synergist 3 of the present invention
[0030] (1) Weigh 13 grams of Atractylodes macrocephala, 16 grams of Codonopsis pilosula, 13 grams of Artemisia annua, 13 grams of myrobalan, 21 grams of pomegranate peel, 18 grams of poplar flower, 12 grams of Alisma, 2 grams of snake gall, 12 grams of ox gall, 45 grams of aloe vera, Rock sugar 30 grams.
[0031] (2) Decoct the raw material twice, add 8 times the amount of water for the first time, decoct for 2 hours, add 6 times the amount of water for the second time, decoct for 1 hour, combine the two decoctions, filter to obtain the filtrate, and concentrate to specific gravity 1.35 extract, add 85-95% edible glacial acetic acid for extraction, recover glacial acetic acid and concentrate, dry at 45-50°C to obtain 16.75 grams of dry extract;
[0032] (3) Grinding the dry extract into powder, adding 20 grams of neomycin, 15 grams of oxytetracycline hydrochloride and 10 grams of sulfam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com