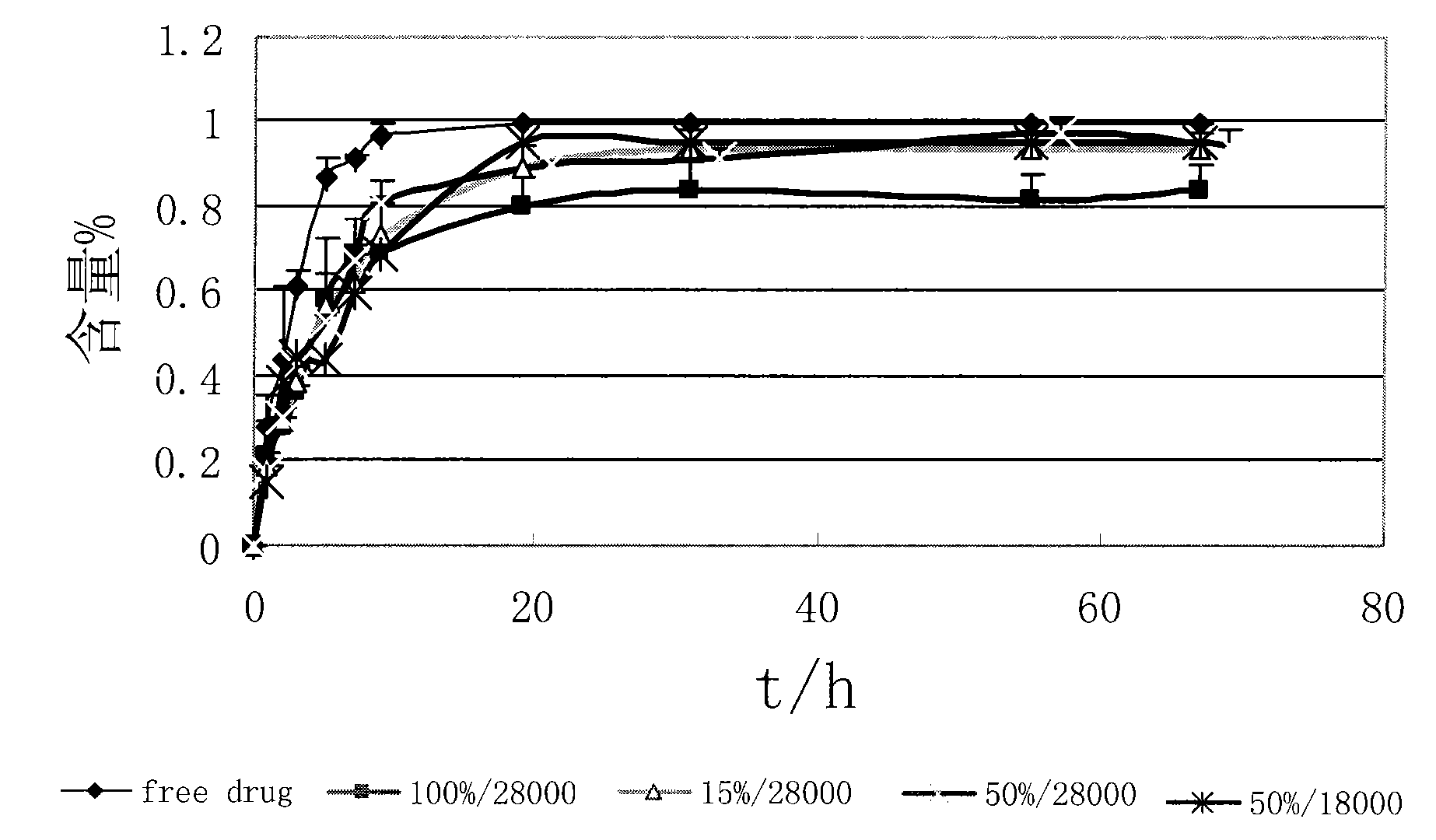
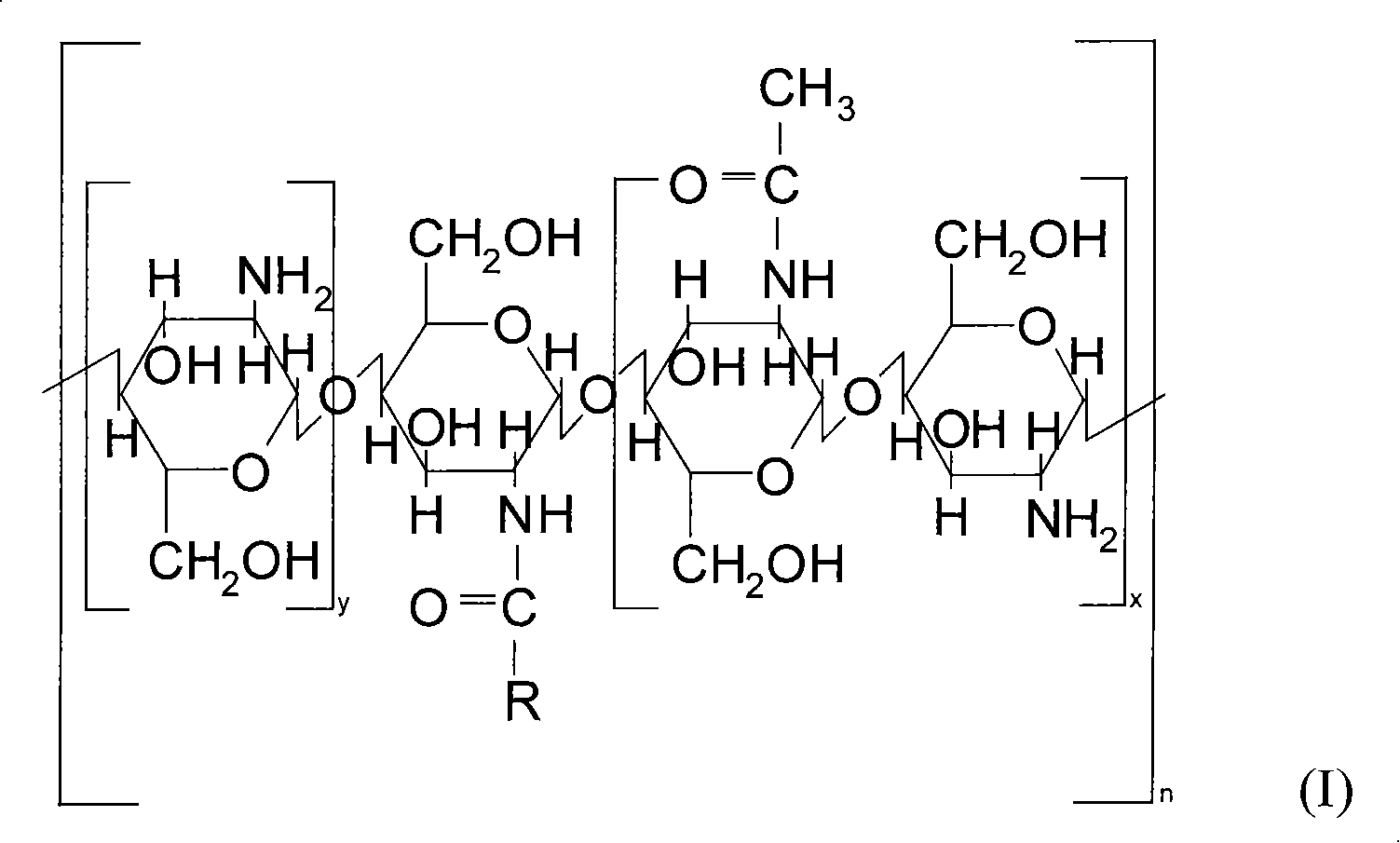
Medicine carrying system of polymer micelle and preparation method thereof
A technology of polymer glue and drug-carrying system, applied in pharmaceutical formulations, anti-tumor drugs, drug combinations, etc., can solve the problems of high viscosity, difficulty in wide application, high acetylation, etc. Prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of chitosan oligosaccharide
[0031] Take commercially available chitosan (70% degree of deacetylation) with a molecular weight of 550kDa, stir for 2 hours at 55°C and pH5. : 100 (w / w) cellulase (produced by Shanghai Boao Biotechnology Co., Ltd.) was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The resulting chitosan degradation solution is filtered to remove impurities, and ultrafiltration is used for fractionation using ultrafiltration membranes with molecular weights of 10 kDa and 30 kDa. The ultrafiltrate with a molecular weight between 10kDa and 30kDa was freeze-dried to obtain chitosan oligosaccharide with a deacetylation degree of 70% and a molecular weight of 18kDa.
[0032] (2) Preparation of salicylic acid-g-chitooligosaccharide graft
[0033]Accurately weigh 0.2756g of carbodiimide (EDC) and 0.0397g of salicylic acid (Salicycleacid, SA) in 3mL of dehydrated ethanol (A solution), then a...
Embodiment 2-4
[0044] Salicylic acid-g-chitooligosaccharide grafts were prepared according to the synthetic recipe shown in Table 1, other conditions were the same as in Example 1, and the test results of physical and chemical properties were shown in Table 2.
[0045] Table 1: The synthetic prescription (n=3) of salicylic acid-g-chitooligosaccharide graft of embodiment 1-4
[0046] Example
Oligochitosan
molecular weight
(kDa)
salicylic acid
theory of
EDC (g)
salicylic acid
(g)
(ml)
Oligochitosan
(g)
(ml)
Example 1
1,8000
50%
0.2756
0.0397
3
0.1
6
Example 2
2,8000
15%
0.0826
0.0119
1
0.1
6
Example 3
2,8000
50%
0.2756
0.0397
3
0.1
6
Example 4
2,8000
100%
0.5512
0.0794
6
0.1
6
...
Embodiment 5-8
[0049] Embodiment 5-8 is loaded with the preparation of paclitaxel micelles
[0050] Accurately weigh 10 mg of the blank salicylic acid-g-chitooligosaccharide carrier material obtained in Examples 1-4 respectively as the raw materials of Examples 5 to 8, and respectively use 10 ml of distilled water to make a 1 mg / ml carrier aqueous solution; Take 10 mg of paclitaxel and dissolve it with 1 ml of ethanol to obtain a 10 mg / ml paclitaxel mother solution. The paclitaxel solution was added to the carrier solution at a drug loading ratio (w / w) of 10%, and the probe was ultrasonicated at 400w for 40 times, working for 2s and resting for 3s. Then the solution was transferred to a dialysis bag (membrane cut-off MWCO=3500) for dialysis to remove ethanol, and the dialysis lasted for 4.5 hours. The dialyzed solution was transferred to a sirloin bottle, and the probe was ultrasonicated again, 400w 40 times, working for 2s and resting for 3s. Centrifuge at 4000rpm for 10min to remove undi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com