Lithium iron phosphate anode material for lithium ion battery and modification method
A technology of lithium iron phosphate and cathode material, which is applied in battery electrodes, electrode manufacturing, chemical instruments and methods, etc., can solve the problems of difficulty in obtaining high-rate electrochemical performance, poor chemical uniformity of products, and deterioration of electrochemical performance, etc. Achieve the effect of solving the inconsistency between preparation conditions and modification conditions, excellent electrochemical performance, and overcoming poor repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Preparation of lithium iron phosphate precursor
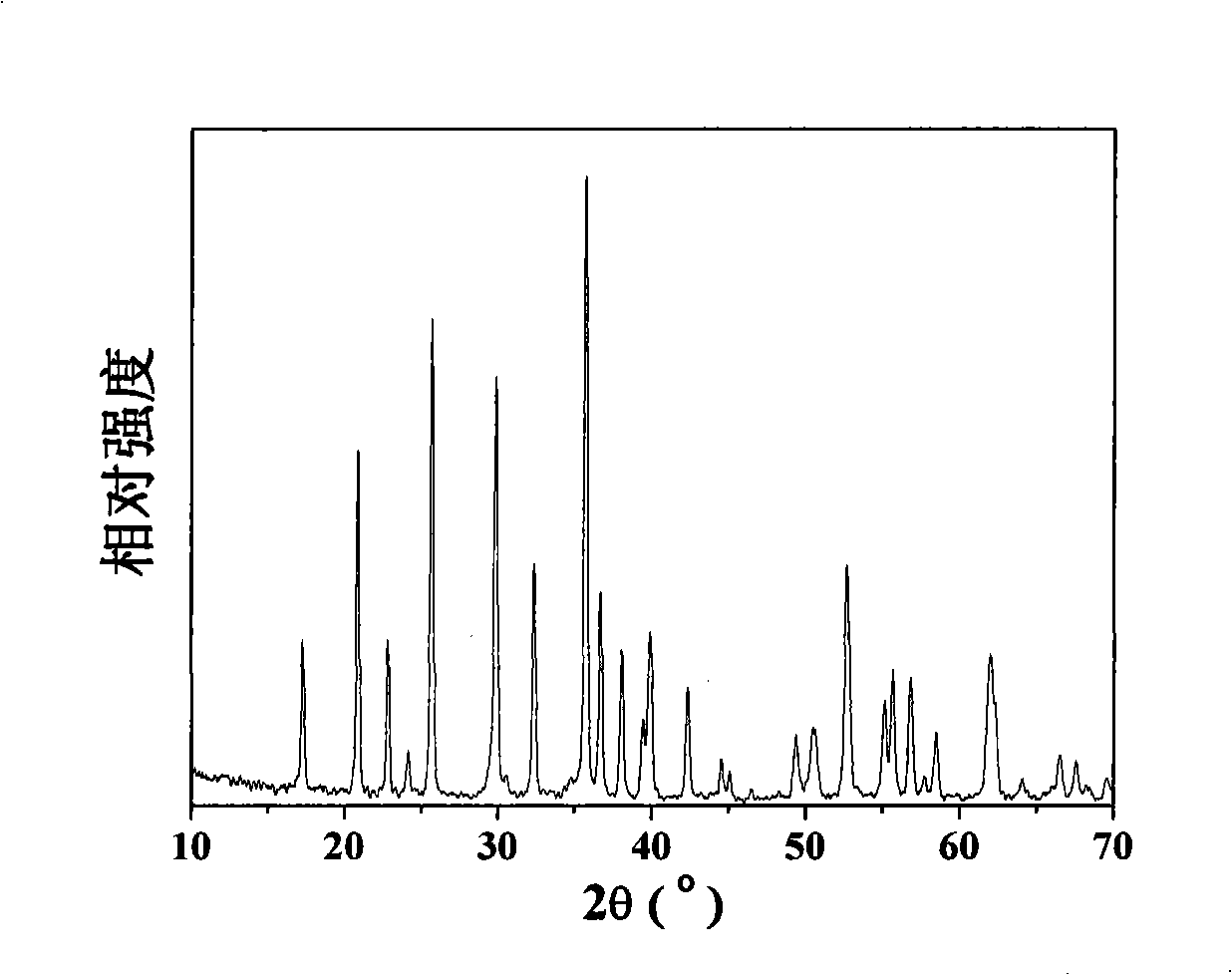
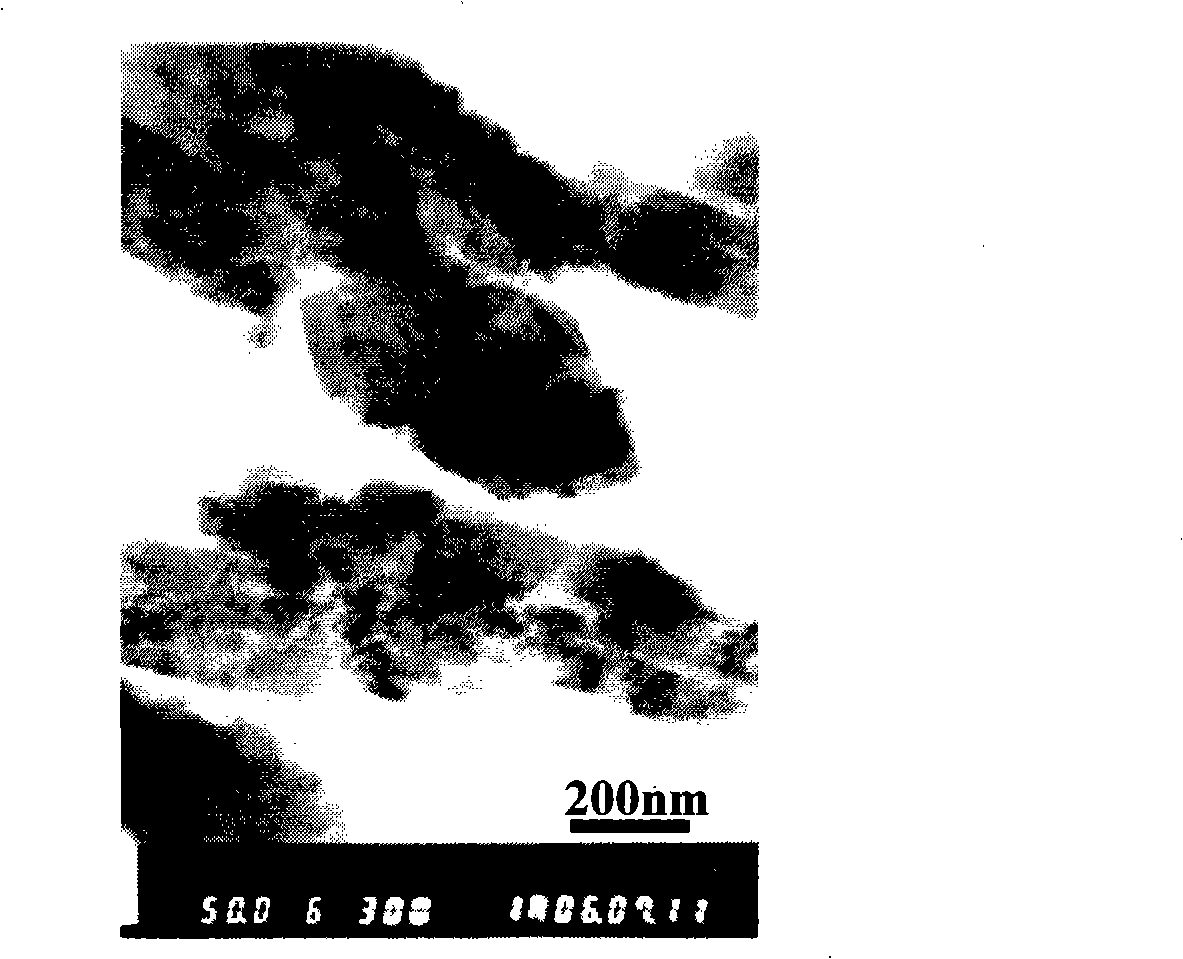
[0038] 34.506g NH 4 h 2 PO 4 (0.3mol) into a 1L autoclave, add 250ml of distilled water to form 1.2mol / L NH 4 h 2 PO 4 solution, then 83.406gFeSO 4 ·7H 2 O (0.3mol) add NH 4 h 2 PO 4 Form uniform milky white suspension in the solution, then 187.5ml LiOH solution (c LiOH =4mol / L) was added in the suspension, gray-green precipitate appeared, stirred vigorously, and after the reaction was complete, 62.5ml Vc solution was added (c Vc =0.32mol / L). Sealed reaction at 210°C and autogenous pressure of 0.8MPa for 12-60 hours, after cooling, the product was washed, filtered, and dried at 60°C to obtain a lithium iron phosphate precursor. X-ray diffraction pattern as figure 1 , indicating that the product is LiFePO 4 , TEM photo as figure 2 , it can be seen that the crystal particles are flakes with a length of about 500 nm.
[0039] 2. Preparation of modified lithium iron phosphate cathode material
[0040] We...
Embodiment 2
[0046] 34.506g NH 4 h 2 PO 4 (0.3mol) into a 1L autoclave, add 250ml of distilled water to form 1.2mol / L NH 4 h 2 PO 4 solution, then 83.406g FeSO 4 ·7H 2 O (0.3mol) add NH 4 h 2 PO 4Form uniform milky white suspension in the solution, then 225ml LiOH solution (c LiOH =4mol / L) was added into the suspension, gray-green precipitate appeared, vigorously stirred, and 25ml Vc solution was added after the reaction was complete (c Vc = 1.2 mol / L). Sealed reaction at 210°C and autogenous pressure of 0.8MPa for 12-60 hours, after cooling, the product was washed, filtered, and dried at 60°C to obtain a lithium iron phosphate precursor.
[0047] Weigh 10g of lithium iron phosphate precursor, 0.3g of acetylene black, 0.135g of Mg(CH 3 COO) 2 After uniform mixing, absolute ethanol was used as a ball milling medium, and the mixture was dried at 80° C. after ball milling for 4-8 hours. Finally, it was calcined at 650°C for 8h. A lithium iron phosphate cathode material coated w...
Embodiment 3
[0050] 41.407g NH 4 h 2 PO 4 (0.36mol) into a 1L autoclave, add 300ml of distilled water to form 1.2mol / L NH 4 h 2 PO 4 solution, and then 100.087g FeSO 4 ·7H 2 O (0.36mol) add NH 4 h 2 PO 4 A uniform milky white suspension was formed in the solution, and then 225ml of 4mol / L LiOH solution (LiOH was 0.9mol) was added to the suspension, a gray-green precipitate appeared, stirred vigorously, and 75ml of Vc solution was added after the reaction was complete (c Vc =0.32mol / L). Sealed reaction at 210°C and autogenous pressure of 0.8MPa for 12-60 hours, after cooling, the product was washed, filtered, and dried at 60°C to obtain a lithium iron phosphate precursor.
[0051] Weigh 10 g of lithium ferrous phosphate, 1.5 g of glucose, 0.14 g of Zn(CH 3 COO) 2 Add 10 mL of distilled water and stir for 10 minutes. Drying at 105°C gave the mixture. The mixture was calcined at 600° C. for 8 hours in nitrogen to obtain a lithium iron phosphate cathode material coated with a con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com