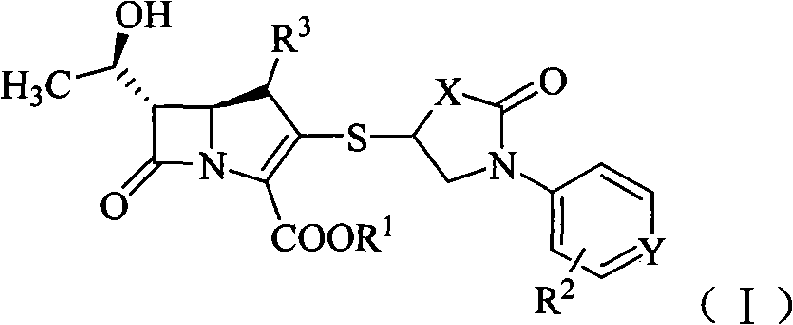
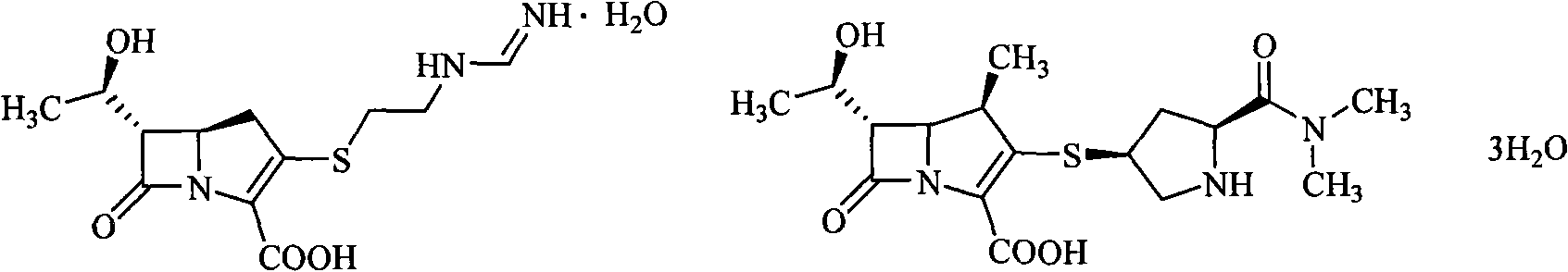
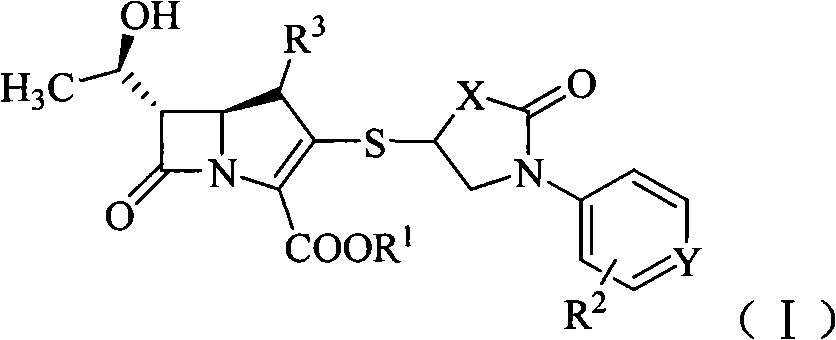
Sulfhydryl oxo heterocycle substituted penem derivates
A technology of alkyl and methyl groups, applied in the field of compositions, can solve the problems of increasing glycopeptide-resistant bacteria, large side effects, increased bacterial resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] The preparation of embodiment 1 (S)-5-mercapto-2-oxo-N-(3-fluorophenyl)-oxazolidine
[0105] In a dry reaction flask, add 16.2g (100mmol) of (S)-5-acetylthio-2-oxooxazolidine, add 150ml of toluene, heat to reflux, then slowly add 17.5g (100mmol) of 3-bromo - 50ml of toluene solution of 1-fluorobenzene, reflux and stir for 6h, after the reaction is completed, cool, add 100ml of water, separate the water layer after stirring, dry the organic layer with anhydrous sodium sulfate, cool to -30°C, slowly add 4.92mol dropwise / L sodium methoxide / methanol solution 40ml, stirred at -20°C for 1h, added 50ml of ice water to dilute, acidified with concentrated hydrochloric acid, separated the water phase, washed the organic phase with water, dried and concentrated, and the residue was treated with ethyl acetate-cyclohexane The alkane was recrystallized to obtain 11.1 g of white solid, yield: 52.1%.
Embodiment 2
[0106] The preparation of embodiment 2 (S)-4-mercapto-2-oxo-N-(3-fluorophenyl-1-yl)-pyrrolidine
[0107] Refer to Example 1 for specific operations, throw (S)-4-acetylthio-2-oxopyrrolidine 15.9g (100mmol), 3-bromo-1-fluorobenzene 17.5g (100mmol), get (S)- 12.7 g of 4-mercapto-2-oxo-N-(3-fluorophenyl-1-yl)-pyrrolidine, yield: 60.2%.
Embodiment 3
[0108] The preparation of embodiment 3 (S)-5-mercapto-2-oxo-N-(2-fluoropyridin-4-yl)-oxazolidine
[0109] Refer to Example 1 for specific operation, throw (S)-5-acetylthio-2-oxooxazolidine 16.2g (100mmol), 4-bromo-2-fluoropyridine 17.6g (100mmol), get (S) - 13.8 g of 5-mercapto-2-oxo-N-(2-fluoropyridin-4-yl)-oxazolidine, yield: 64.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com