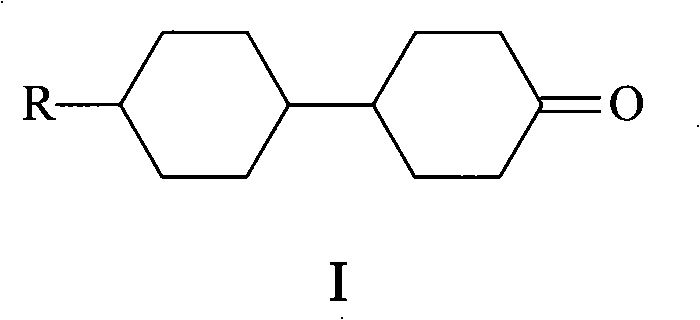
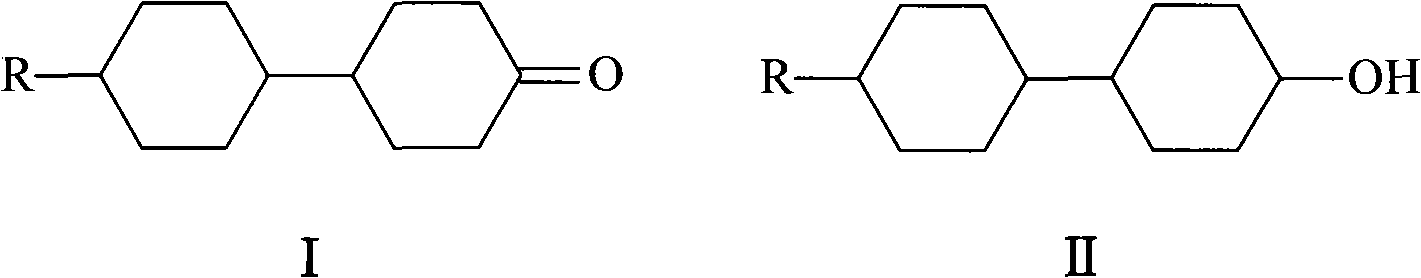Method for synthesizing 4-(4'-n-alkyl cyclohexyl)cyclohexanone
A synthesis method and the technology of alkyl rings, which are applied in the field of synthesis of important liquid crystal intermediate 4-cyclohexanone, can solve the problems of serious environmental pollution and low yield, and achieve low oxidation cost, low price and simple synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Add 2.3g (0.007mol) of sodium tungstate dihydrate, 2.0g (0.0007mol) of phosphotungstic acid, and 34g of hydrogen peroxide (30% by mass) into a 250mL three-necked flask with a reflux condenser, and stir for 15 minutes , the solution changed from yellow to pale yellow. Add 22.4 (0.1 mol) g of 4-(4'-n-propylcyclohexyl) cyclohexanol (II) and 144 g of N-methylpyrrolidone to the above reaction system, and heat to keep the system temperature at 90°C. After 5 hours, the reaction was analyzed by gas chromatography, and the heating was turned off. Change the reaction device to a vacuum distillation device, distill N-methylpyrrolidone and cool to room temperature, then extract with petroleum ether, dry over anhydrous sodium sulfate, filter to remove the desiccant, and obtain 4-(4'-n- Propylcyclohexyl)cyclohexanone (I) 20.2g, yield 91.0%.
[0014] IR(KBr)v / cm -1 : 2954, 2919, 2850, 1719, 1464, 1448GC-MS (EI): 222 (M + ), 204, 166
[0015] 13 C NMR, δ C : 215.10, 43.42, 42.81...
Embodiment 2
[0017] Other conditions are the same as in Example 1, and the temperature of the heating system is maintained at 80°C. After 8 hours, the reaction was analyzed by gas chromatography to obtain 20.0 g of 4-(4'-n-propylcyclohexyl)cyclohexanone with a yield of 90.1%.
Embodiment 3
[0019] Other conditions were the same as in Example 1, 23.8 (0.1 mol) g of 4-(4'-n-butylcyclohexyl)cyclohexanol was added to obtain 22.1 g of 4-(4'-n-butylcyclohexyl)cyclohexanone with a yield of 93.6%.
[0020] IR(KBr)v / cm -1 : 2951, 2918, 2855, 1717, 1466, 1450GC-MS (EI): 236 (M + ), 218, 180
[0021] 13 C NMR, δ C : 216.80, 43.54, 32.81, 44.51, 45.11, 33.06, 36.90, 40.77, 40.07, 32.14, 25.83, 16.26
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com