Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
A sustained-release preparation, cilostazol technology, applied in the direction of organic non-active ingredients, oil/fat/wax non-active ingredients, powder delivery, etc., can solve the problem of drug or additive stability damage, wax matrix particle formulation technology There are no reports, solidification and other problems, to achieve high biological availability, effective pharmacological effects, and ensure the effect of sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
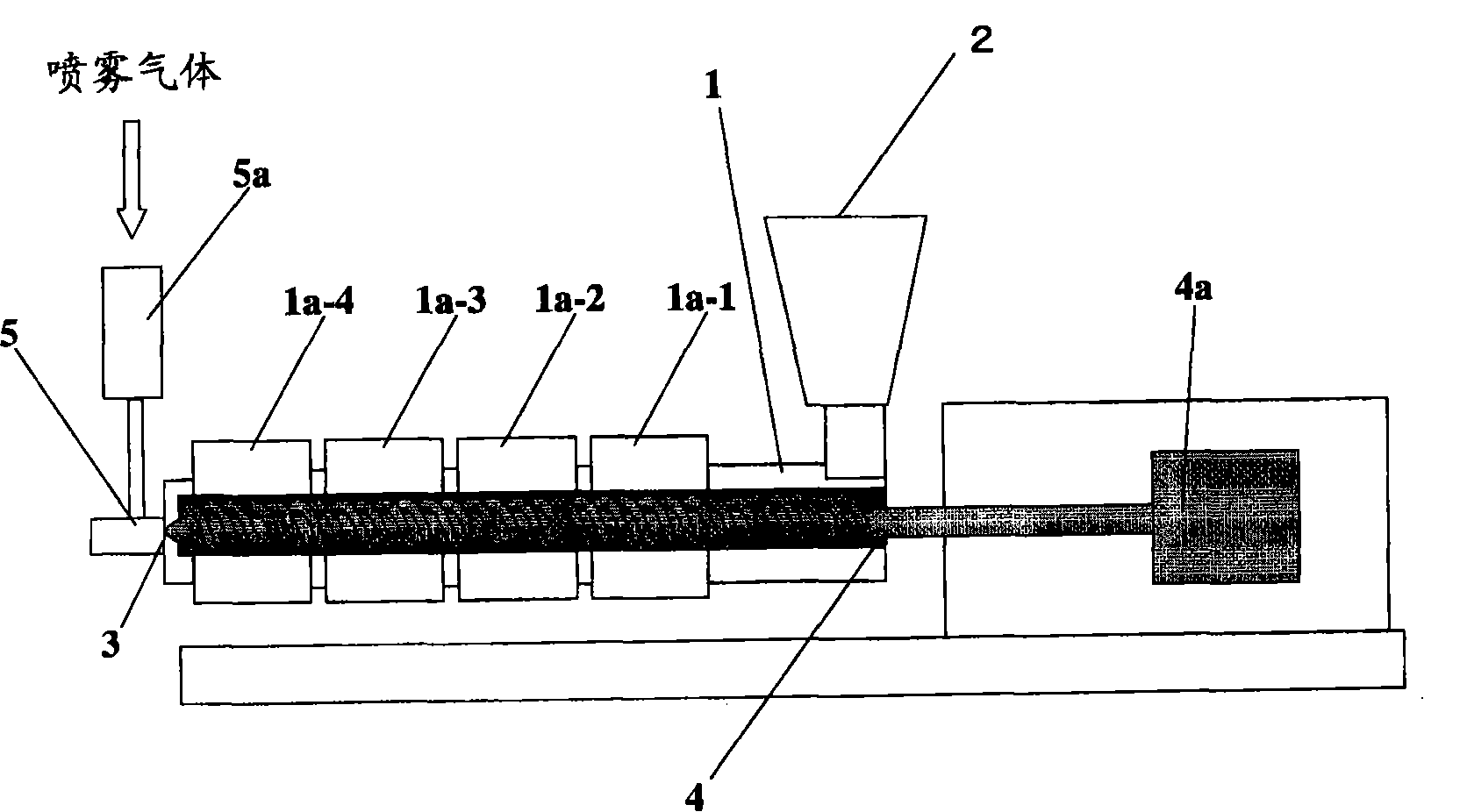
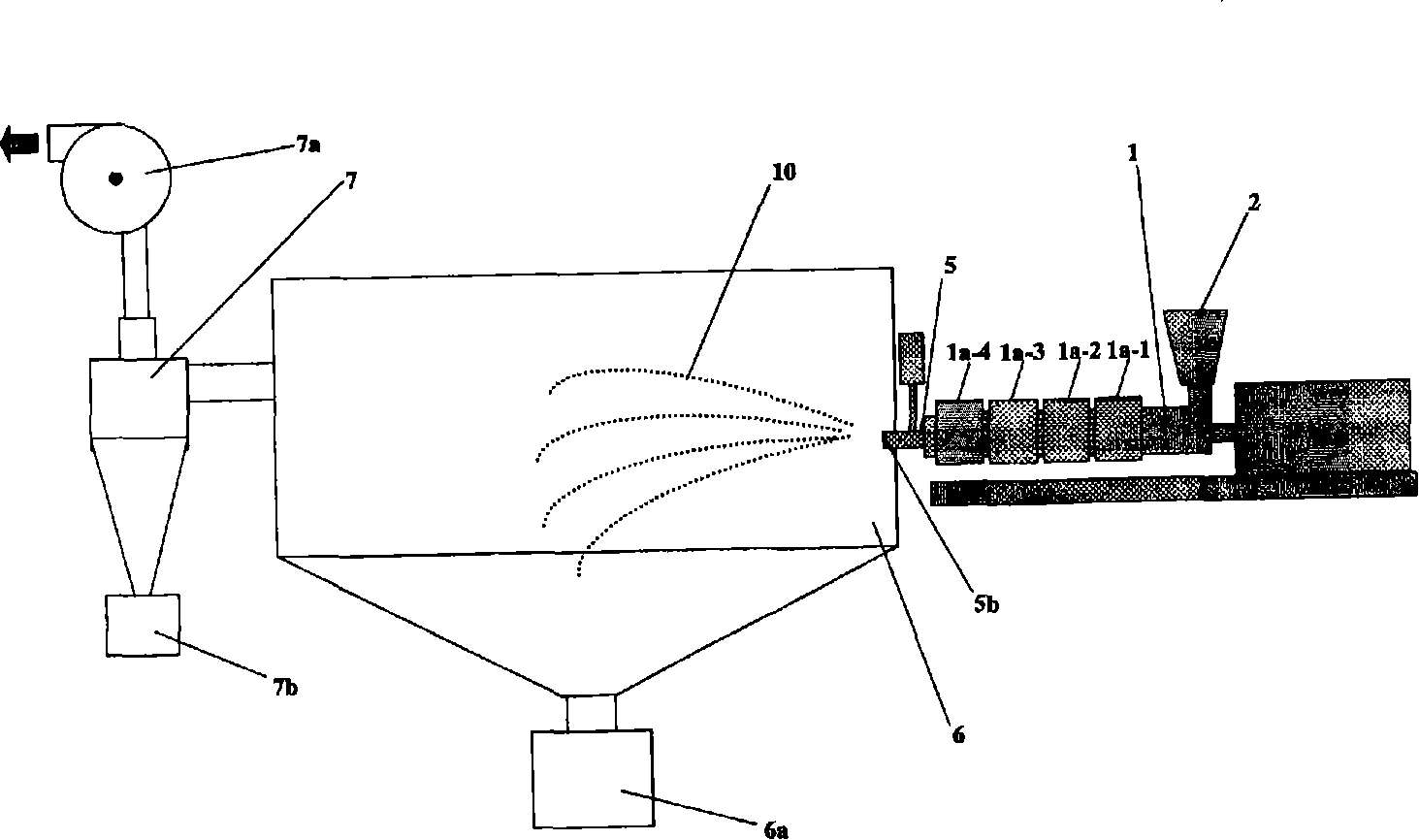
[0152] use figure 2 The extruder of the configuration shown, produces wax matrix particles. As an extruder, the structure and its operating conditions are shown below.
[0153] Extruder type: Twin-screw extruder (KEX-25, manufactured by Kurimoto Iron Works)
[0154] Screw shape: From the downstream side to the upstream side, the shape in which the conveying, kneading, and mixing sections are connected in sequence
[0155] Nozzle: Dual Fluid Nozzle
[0156] Screw part length: Approximately 50cm
[0157] Rotation speed of the screw: 125rpm
[0158] The shape and diameter of the discharge hole of the nozzle: circular, 0.5mm
[0159] The residence time of raw materials in the drum: about 2 minutes
[0160] Drum temperature setting temperature: drum sleeve 1a-1 is 140°C, drum sleeve 1a-2 is 150°C, drum sleeve 1a-3 and 1a-4 are 160°C
[0161] Temperature and introduction speed of spray gas: about 200°C, 25L / min
[0162] Discharge speed per discharge hole of molten kneaded...
Embodiment 2
[0168] As raw materials, 300 g of theophylline, 10 g of ethyl cellulose, and 690 g of fatty acid glyceride (glycerol monobehenate; melting point of about 75° C.) were mixed, and wax was produced under the same conditions as in Example 1 above using the obtained mixed raw material. matrix particles.
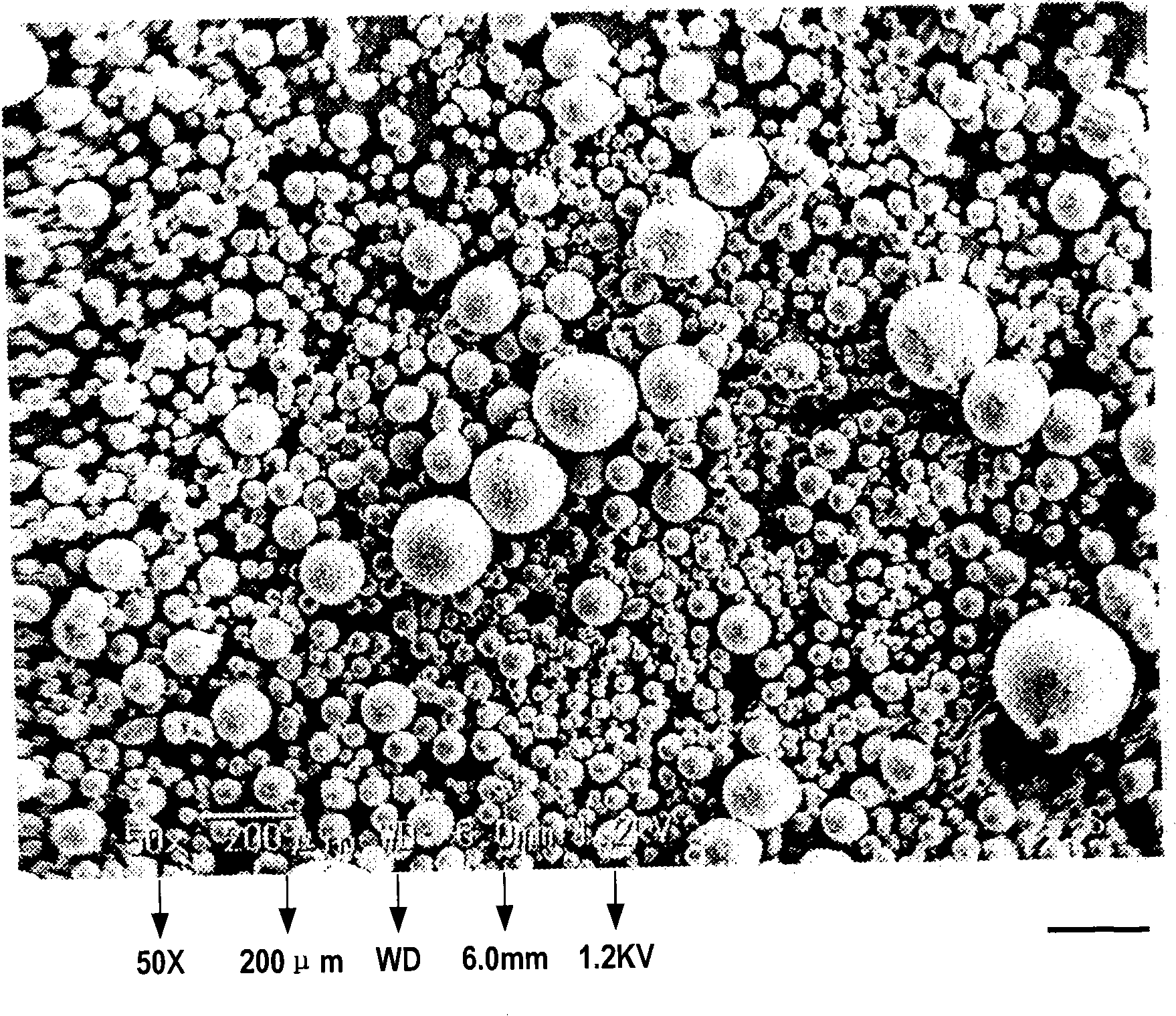
[0169] The obtained wax matrix particles were spherical, and when the particle size distribution was measured with a laser diffraction particle size distribution meter (Tohnichi Computer Application), the 10% cumulative diameter was 43 μm, the 50% cumulative diameter (average particle diameter) was 88 μm, and the 90% cumulative diameter was 160 μm, 99% cumulative diameter is 204 μm.
[0170] In addition, it was confirmed that no troubles such as theophylline precipitation in the extruder and liquid clogging occurred during the production process. In addition, the content of theophylline in the obtained wax-based particles was about 100% relative to the theoretical value.
Embodiment 3
[0172] As raw materials, 300 g of theophylline and 700 g of hardened oil (melting point: about 86° C.) were mixed, and using the obtained mixed raw material, wax matrix particles were produced under the same conditions as in Example 1 above. The obtained wax matrix particles were spherical, and when the particle size distribution was measured with a laser diffraction particle size distribution meter (Tohnichi Computer Application), the 10% cumulative diameter was 48 μm, the 50% cumulative diameter (average particle diameter) was 96 μm, and the 90% cumulative diameter was 169 μm, 99% cumulative diameter is 221 μm.
[0173] In addition, it was confirmed that no troubles such as theophylline precipitation in the extruder and liquid clogging occurred during the production process. In addition, the content of theophylline in the obtained wax-based particles was about 100% relative to the theoretical value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com