Method for recycling pure acid from metallic ion containing waste acid and regenerating alkali
A metal ion, acid recovery technology, applied in the direction of chlorine/hydrogen chloride purification, sulfur trioxide/sulfuric acid, chlorine/hydrogen chloride, etc., can solve the problems of alkali consumption, waste of water resources, difficulty in recycling, etc., and achieve production cost and energy consumption. Low, no secondary pollution, high acid recovery effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
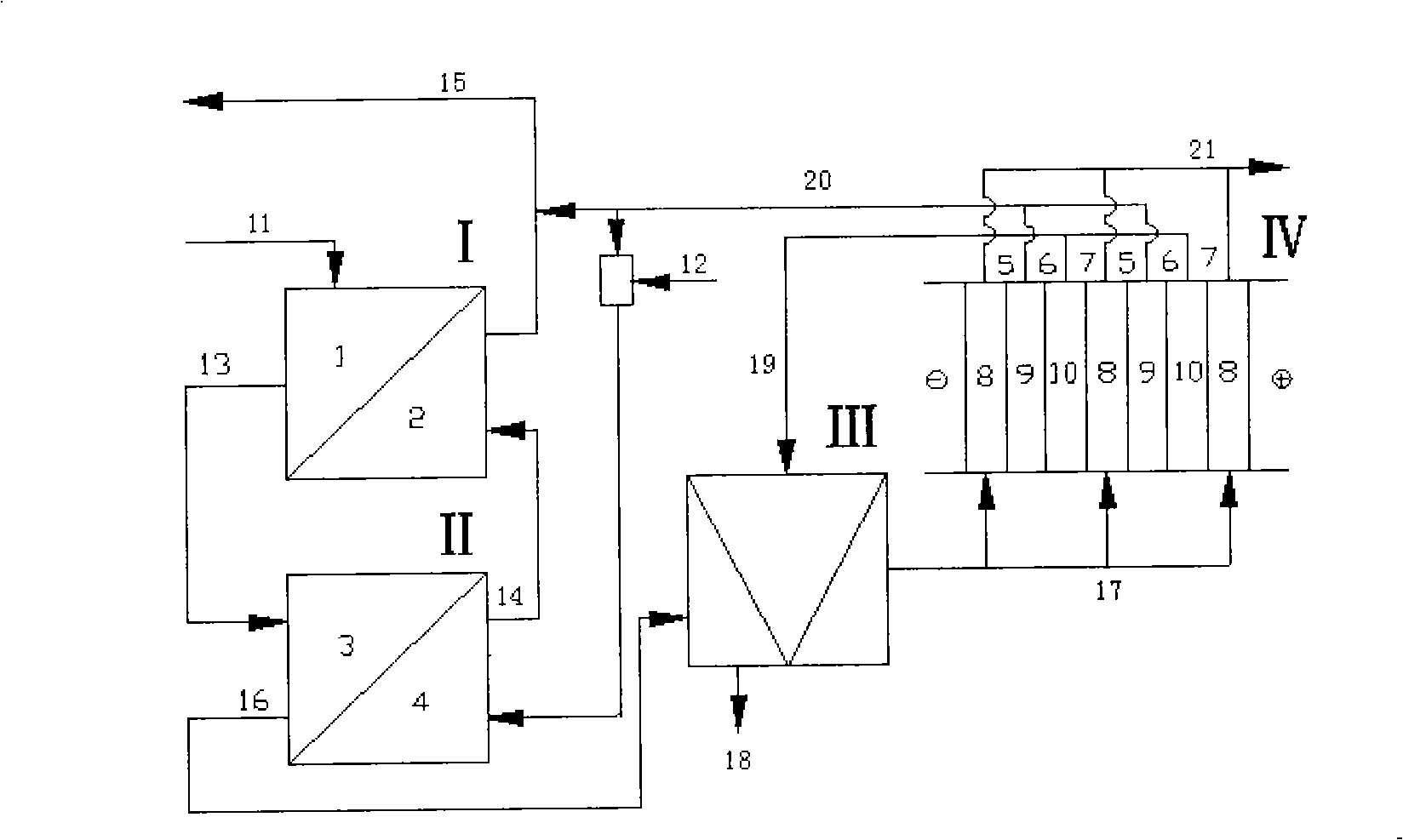
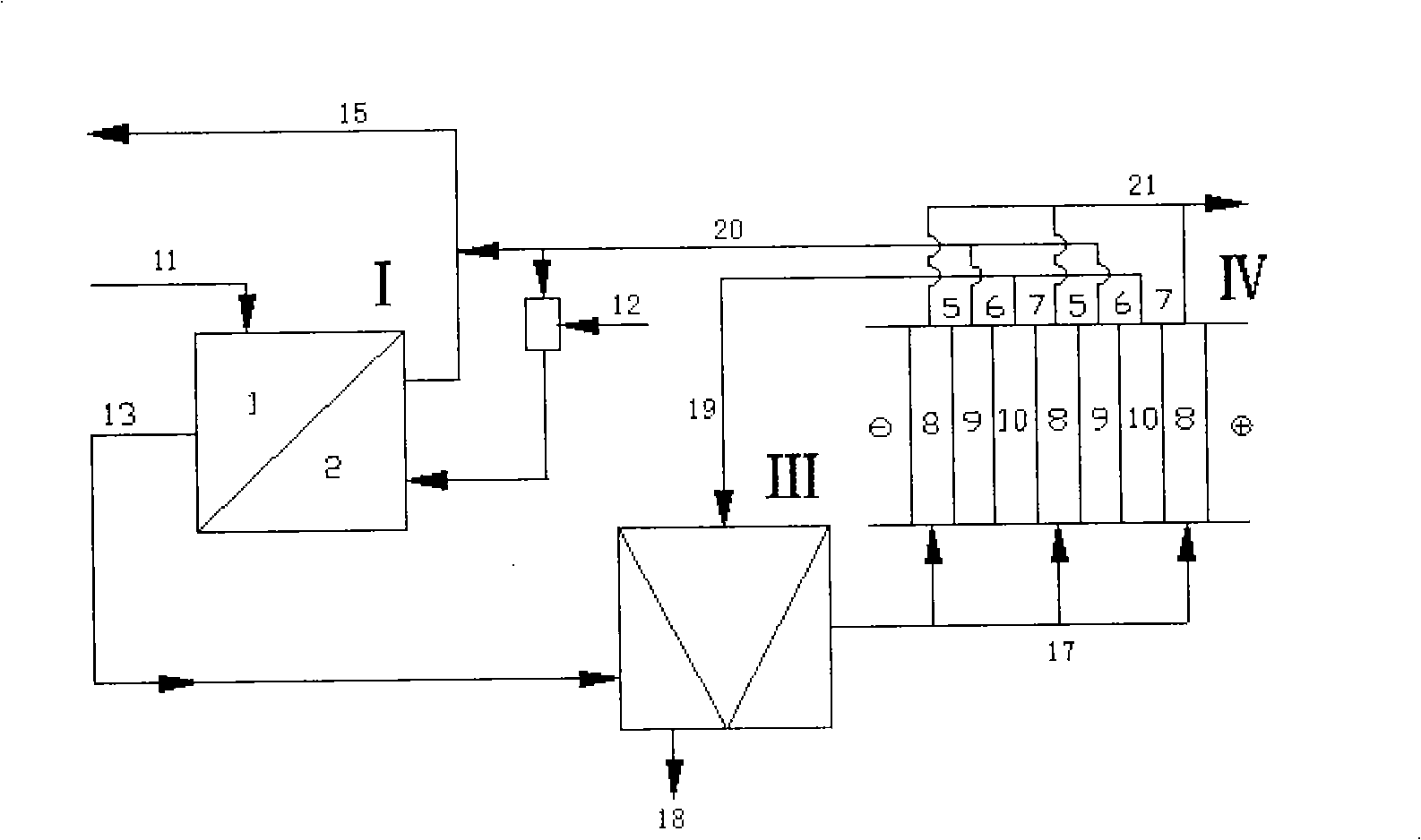
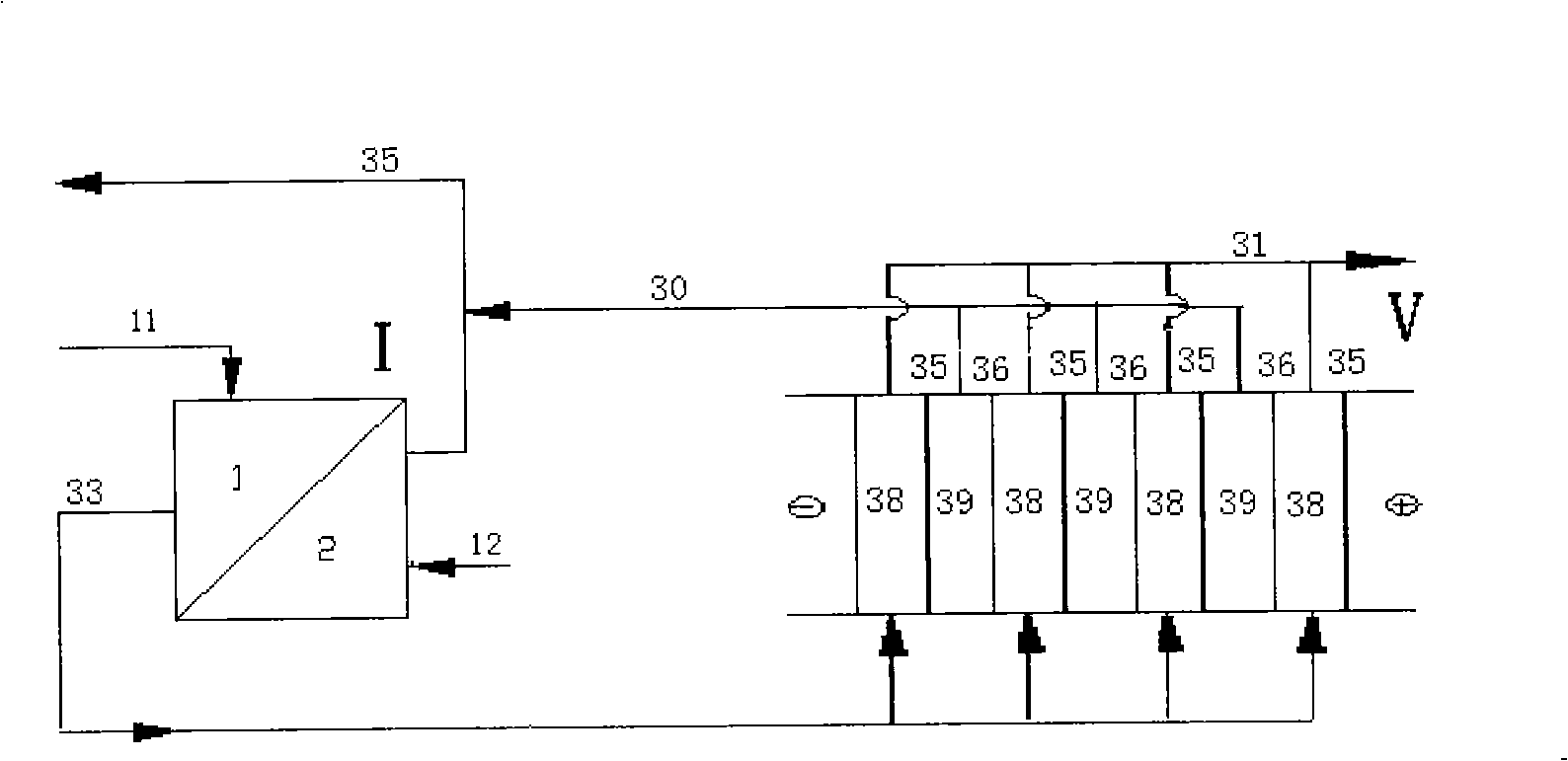
[0011] Specific implementation mode one: the step of regeneration of pure acid from waste acid containing metal ions in this embodiment is as follows (see figure 1 ): (a) first enter the waste acid 11 into the first-stage diffusion dialysis tank I, so that the volume ratio of the waste acid to the collected liquid in the first-stage acid recovery chamber (which can be the secondary recovery acid 14) is 1:0.5~1: 3.0, the flow rate of the waste acid liquid 11 is 0.2-0.6L / h, and the flow rate of the collected liquid in the acid recovery chamber is 0.1-1.8L / h; (b) the first-level raffinate 13 after step a enters the second-level diffusion In the dialysis tank II, the volume ratio of the primary raffinate 13 to the collected liquid in the secondary acid recovery chamber (the mixed solution of tap water 12 and regenerated acid 20) is 1:0.5 to 1:3.0, and the flow rate of the primary raffinate 13 is 0.2 ~0.6L / h, the flow rate of the collected liquid in the secondary acid recovery cham...
specific Embodiment approach 2
[0012] Embodiment 2: In this embodiment, in step d, the form of bipolar membrane electrodialysis adopts three compartments (cathode membrane-bipolar membrane-cation membrane alternately arranged). Others are the same as in the first embodiment.
specific Embodiment approach 3
[0013] Specific embodiment three: the embodiment recycles the battery factory into sulfuric acid waste liquid, which is composed of sulfuric acid concentration of 12%, ferrous ion concentration of 15 mg / L, and lead ion concentration of 3 mg / L. The flow rate of the waste sulfuric acid liquid 11 is 0.42L / h; the flow rate of the collected liquid 14 entering the acid recovery chamber of the primary diffusion dialysis tank is 0.84L / h; the electrodialysis is performed under the condition of a voltage of 10V for 3h. Other steps are the same as in the first embodiment. The experimental results are shown in Table 1 and Table 2.
[0014] Table 1 Effects of acid recovery and metal ion retention in two-stage diffusion dialysis
[0015]
[0016] * The data in ( ) represents the total sulfuric acid recovery rate R of two-stage diffusion dialysis, and its calculation formula is:
[0017] R=[(the sulfuric acid content in the recovery acid 15+the sulfuric acid content in the recovery aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com