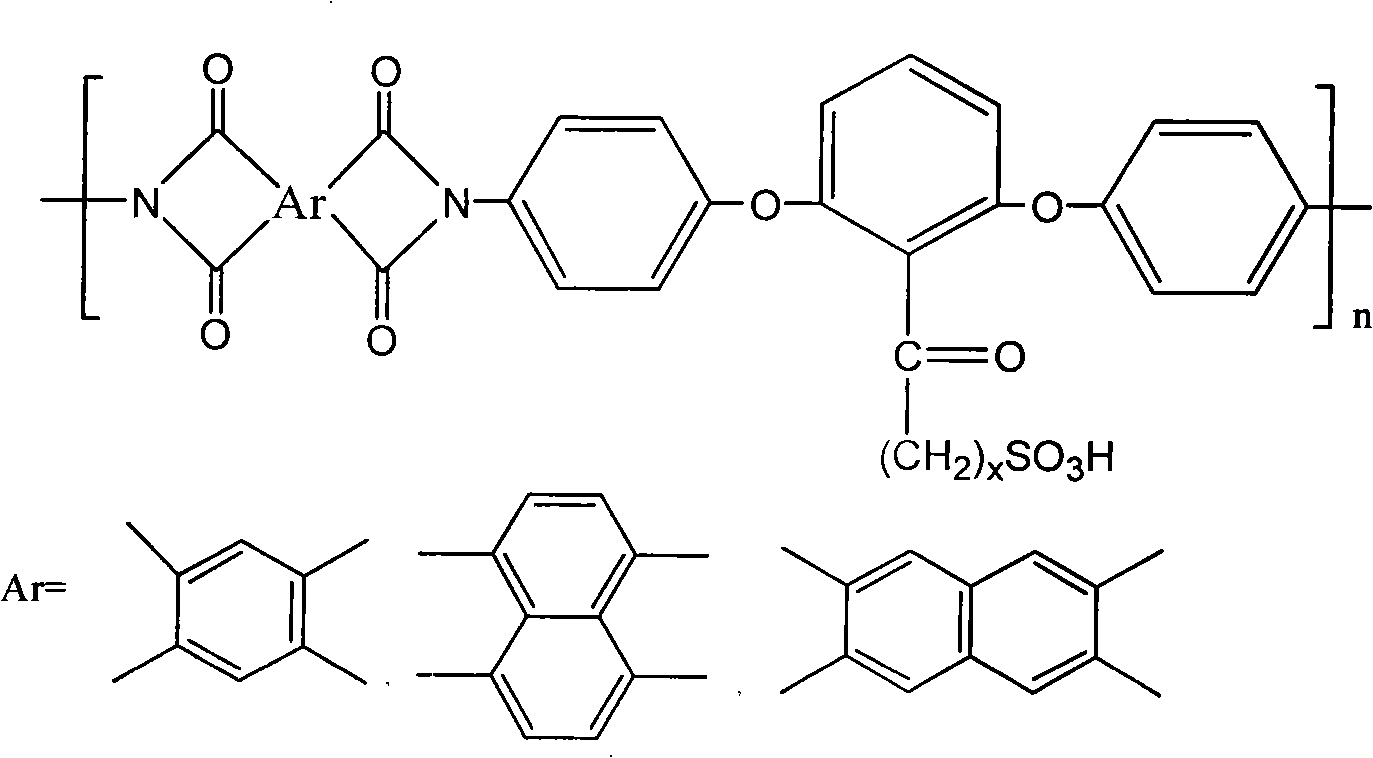
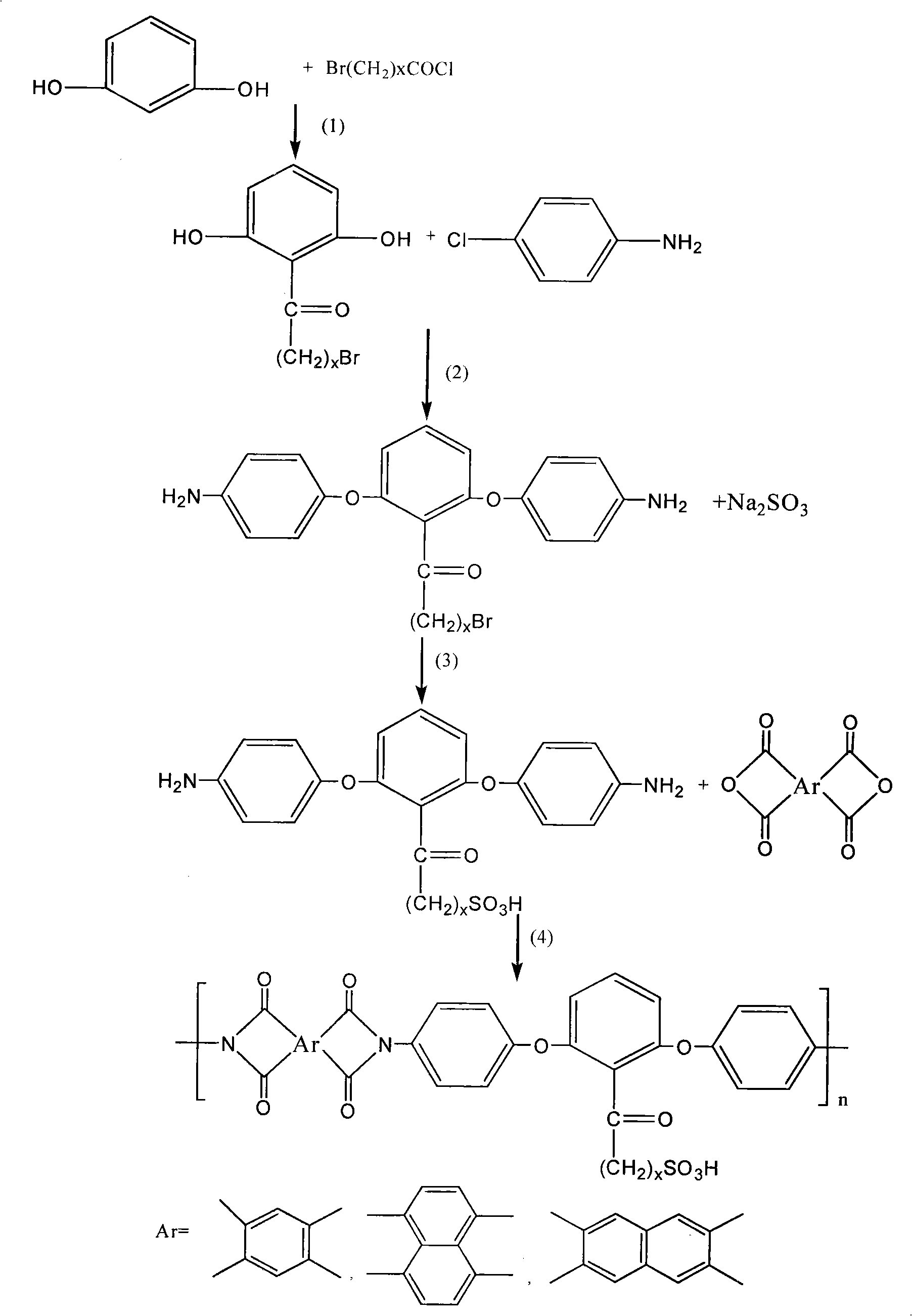
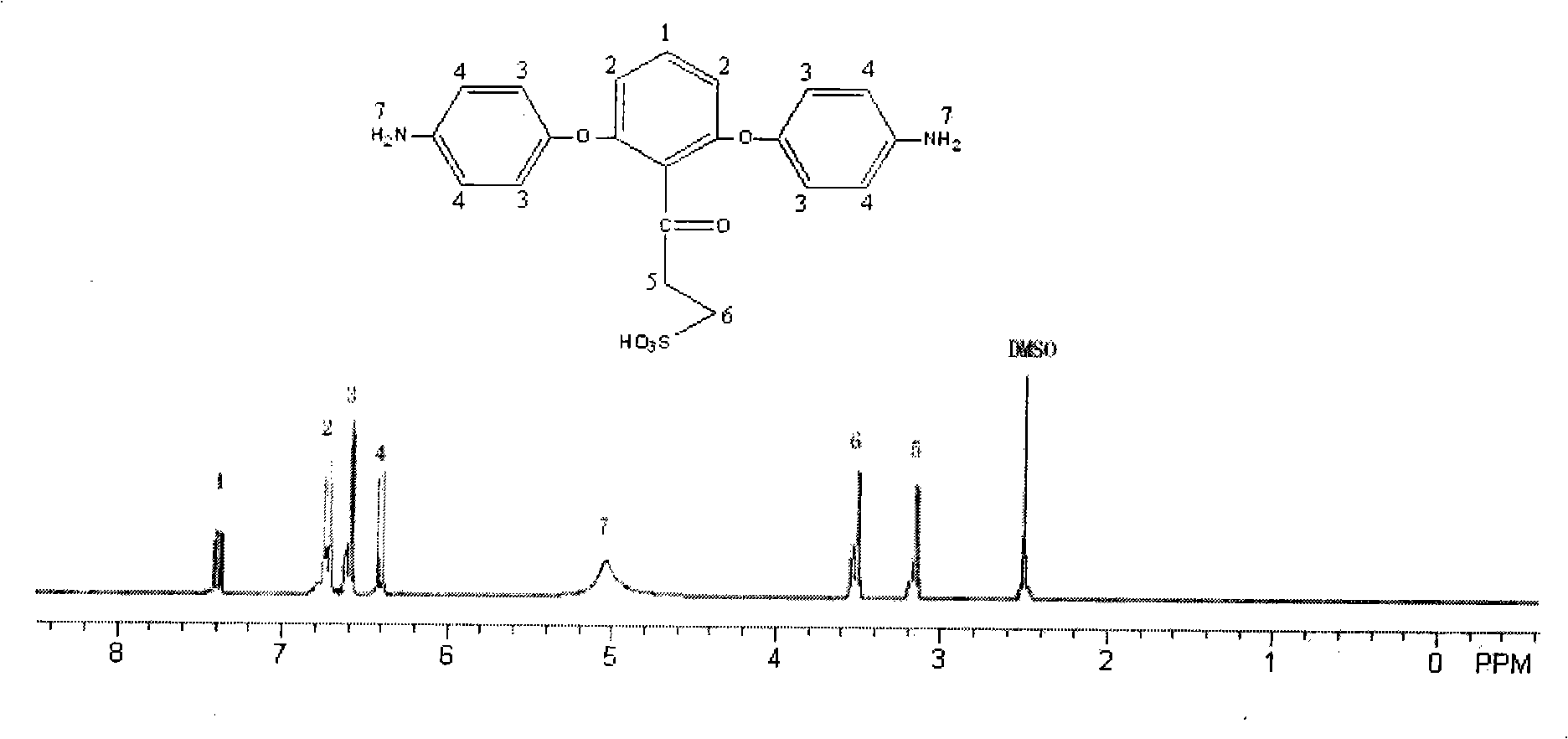
Low-swelling sulfonation polyimide proton exchanging membrane and preparation thereof
A technology of sulfonated polyimide and proton exchange membrane, applied in the field of low-swelling sulfonated polyimide proton exchange membrane and its preparation, can solve the problem of increasing methanol permeability, reducing fuel utilization efficiency, reducing proton exchange membrane mechanical In order to improve the fuel utilization rate, enhance the high temperature swelling stability and reduce the permeability of methanol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Dissolve 11g (0.1mol) of resorcinol monomer and 14.6g (0.11mol) of aluminum trichloride in 100g of dichloromethane, cool to 0°C with an ice bath under a nitrogen atmosphere, and stir for 10 minutes , then 17.15g (0.1mol) 3-bromopropionyl chloride was added dropwise within 15 minutes, stirred at 0°C for 4 hours, after the reaction was completed, the reaction product was poured into 100g of ice water containing 10g of concentrated hydrochloric acid, and the Separate the organic phase, then wash once with 100g10% sodium hydroxide, then wash twice with 100g deionized water, and finally dry with 20g anhydrous magnesium sulfate, after filtering, distill off most of the solvent in the mixed solution, and use 30g The sherwood oil recrystallization obtains 20.8g (productive rate 85%) to have the diphenol monomer of bromopropyl group branch;
[0031] (2) the above-mentioned obtained diphenol monomer with bromopropyl branched chain gets 24.5g (0.1mol) and 25.5g (0.2mol) of 4-c...
Embodiment 2
[0037] (1) Dissolve 11g (0.1mol) of resorcinol monomer and 14.6g (0.11mol) of aluminum trichloride in 100g of dichloromethane, cool to 0°C with an ice bath under a nitrogen atmosphere, and stir for 10 minutes , then 19.95g (0.1mol) 5-bromovaleryl chloride was added dropwise within 15 minutes, stirred at 0°C for 4 hours, after the reaction was completed, the reaction product was poured into 100g of ice water containing 10g of concentrated hydrochloric acid, and the Separate the organic phase, then wash once with 100g10% sodium hydroxide, then wash twice with 100g deionized water, and finally dry with 20g anhydrous magnesium sulfate, after filtering, distill off most of the solvent in the mixed solution, and use 30g The sherwood oil recrystallization obtains 22.1g (productive rate 81%) to have the diphenol monomer of bromopentyl branch;
[0038] (2) the above-mentioned obtained diphenol monomer with bromopentyl branch gets 27.3g (0.1mol) and 25.5g (0.2mol) of 4-chloroaniline, an...
Embodiment 3
[0044] (1) Dissolve 11g (0.1mol) of resorcinol monomer and 14.6g (0.11mol) of aluminum trichloride in 100g of dichloromethane, cool to 0°C with an ice bath under a nitrogen atmosphere, and stir for 10 minutes , then 21.35g (0.1mol) 6-bromohexanoyl chloride was added dropwise within 15 minutes, stirred at 0°C for 4 hours, after the reaction was completed, the reaction product was poured into 100g of ice water containing 10g of concentrated hydrochloric acid, and the Separate the organic phase, then wash once with 100g10% sodium hydroxide, then wash twice with 100g deionized water, and finally dry with 20g anhydrous magnesium sulfate, after filtering, distill off most of the solvent in the mixed solution, and use 30g The sherwood oil recrystallization obtains 25.8g (90% of productive rate) the diphenol monomer that has bromohexyl branch;
[0045] (2) get the 4-chloroaniline of 28.7g (0.1mol) and 25.5g (0.2mol) of the diphenol monomer with bromohexyl branch obtained above, the sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Methanol permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com