Method for producing polyaniline compound
A compound and dye compound technology, applied in the preparation of amino compounds from amines, the preparation of organic compounds, organic chemical methods, etc., can solve problems such as difficulty in applying large-scale systems and expensive production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: preparation compound (III-1)
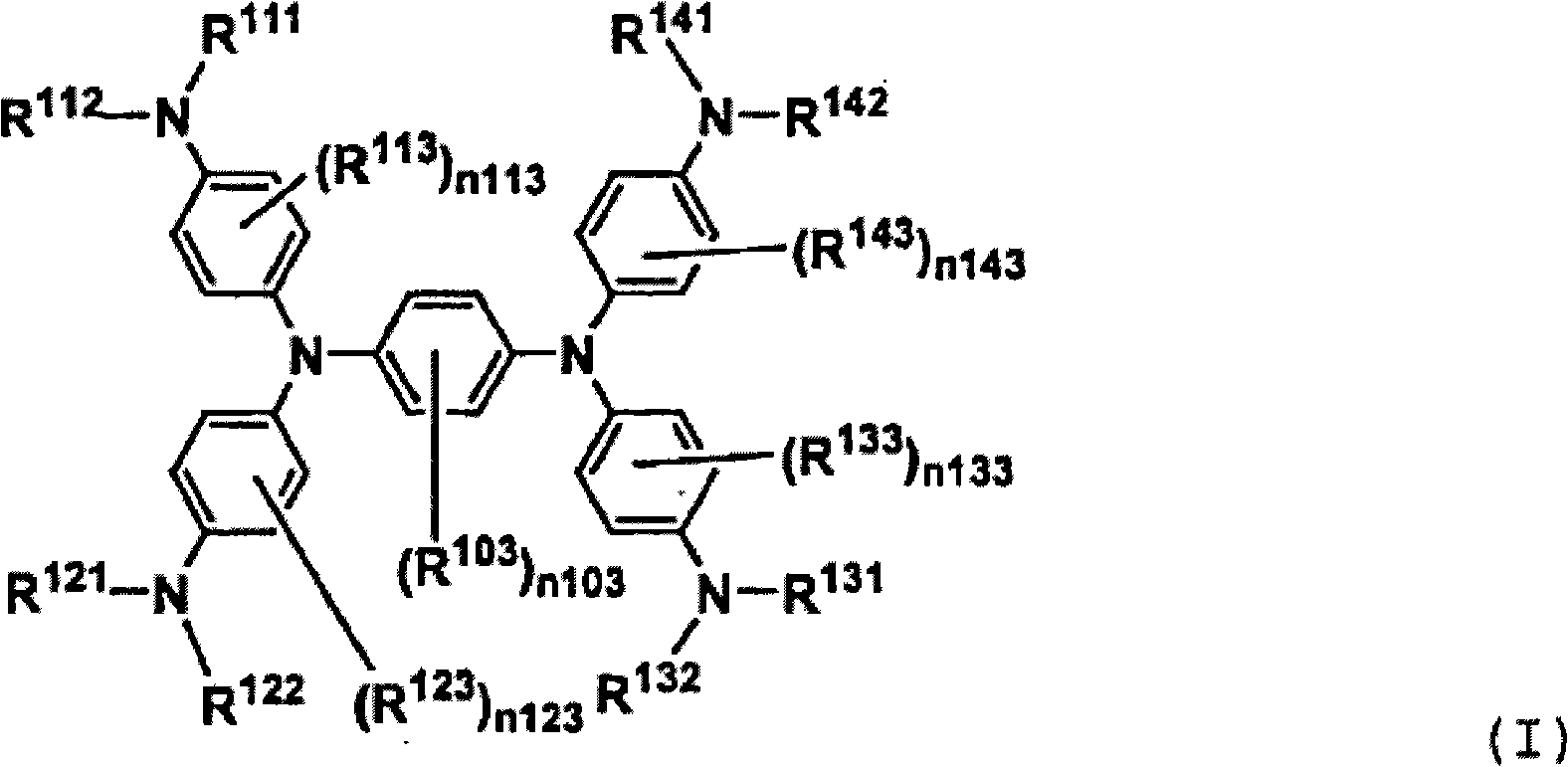
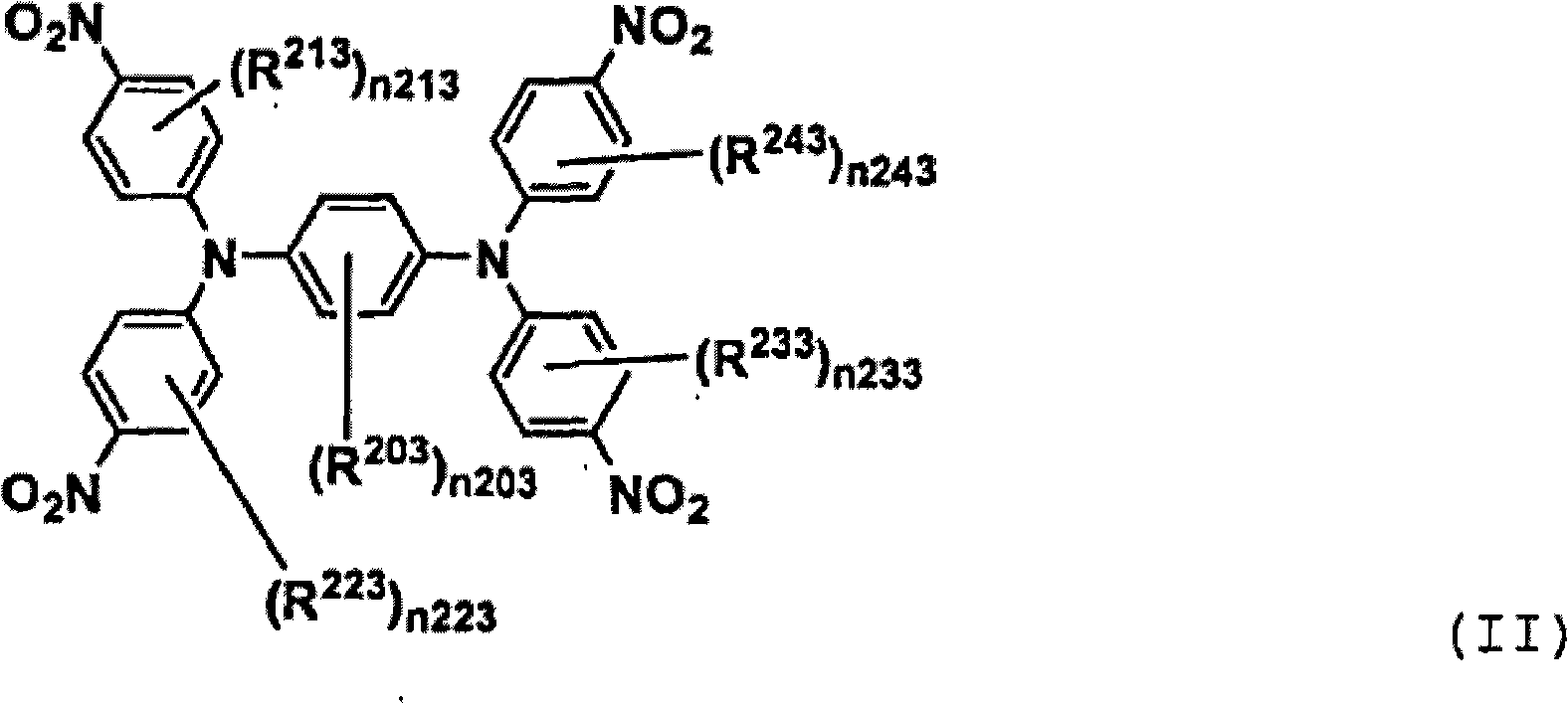
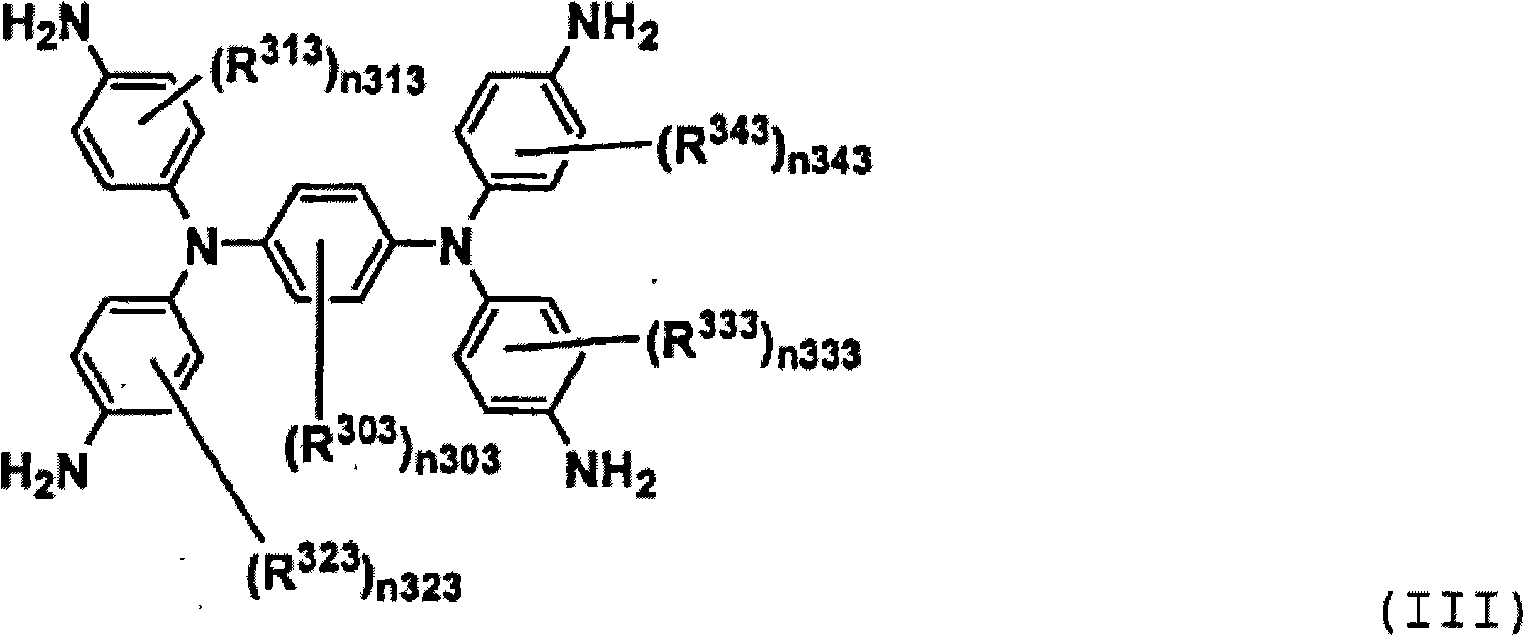
[0076] Compound (III-1) was prepared according to the following scheme.
[0077]
[0078] Compound (II-1) Compound (III-1)
[0079] The solution of 160g compound (II-1), the solution of 0.32g iron chloride (III) and 160ml Virahol, and 8.1g gac and 1340ml 1-methyl-2-pyrrolidone are put into three-necked flask, and Heat with stirring until an internal temperature of 100°C. 450 g of hydrazine monohydrate (80% aqueous solution) was added dropwise thereto over 1 hour, then it was kept heated and stirred at an internal temperature of 100-110° C. for 5 hours, and then cooled to room temperature. It was filtered to remove activated carbon, then 800 ml of methanol and 1200 ml of water were continuously added dropwise thereto, and the resulting crystals were taken out by suction filtration to obtain 125.2 g of expected compound (III-1) (yield, 98%). Its mass spectrum gave M / e=472.
Embodiment 2
[0080] Embodiment 2: preparation compound (III-1)
[0081] The solution of 160g compound (II-1), 1.07g ferric chloride (III) 6-hydrate and 160ml Virahol, and 16.2g gac and 1500mlsulforane are put into three-necked bottle, and heat while stirring until 100 °C internal temperature. 450 g of hydrazine monohydrate (80% aqueous solution) was added dropwise thereto over 1 hour, then it was kept heated and stirred at an internal temperature of 100-110° C. for 5 hours, and then cooled to room temperature. It was filtered to remove activated carbon, then 800 ml of methanol and 1200 ml of water were continuously added dropwise thereto, and the obtained crystals were taken out by suction filtration to obtain 122.0 g of expected compound (III-1) (yield, 96%). Its mass spectrum gave M / e=472.
Embodiment 3
[0082] Embodiment 3: preparation compound (III-1)
[0083] Put 160g of compound (II-1), 1.55g of iron oxide (III), 8.1g of activated carbon, 1000ml of N,N-dimethylacetamide and 100ml of water into a three-necked bottle, and heat it up to 100°C while stirring internal temperature. 600 g of hydrazine monohydrate (80% aqueous solution) was added dropwise thereto over 2 hours, then it was kept heated and stirred at an internal temperature of 100-115° C. for 5 hours, and then cooled to room temperature. It was filtered to remove activated carbon, then 800 ml of methanol and 1200 ml of water were continuously added dropwise thereto, and the resulting crystals were taken out by suction filtration to obtain 117.6 g of expected compound (III-1) (yield, 92%). Its mass spectrum gave M / e=472.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com