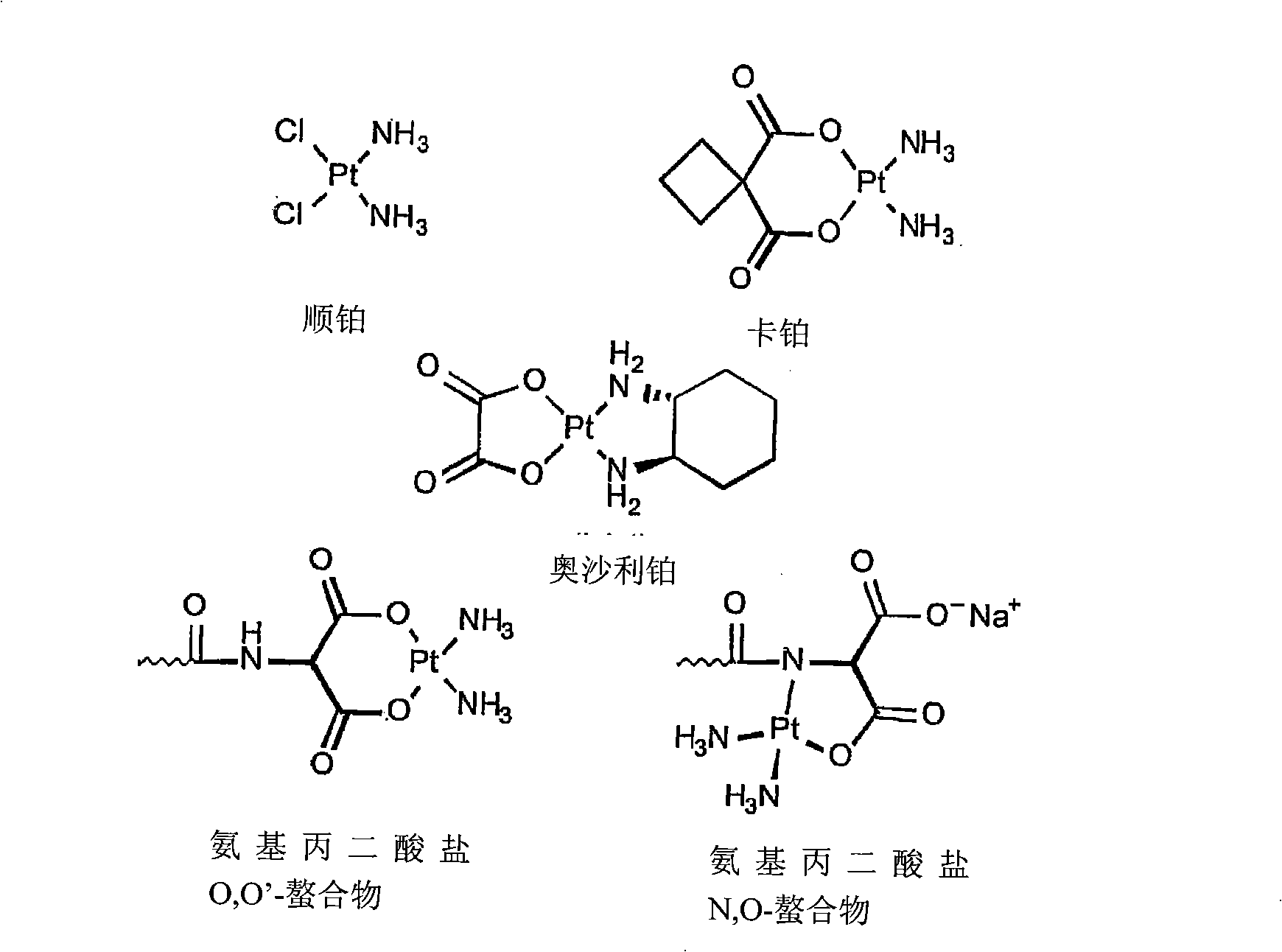
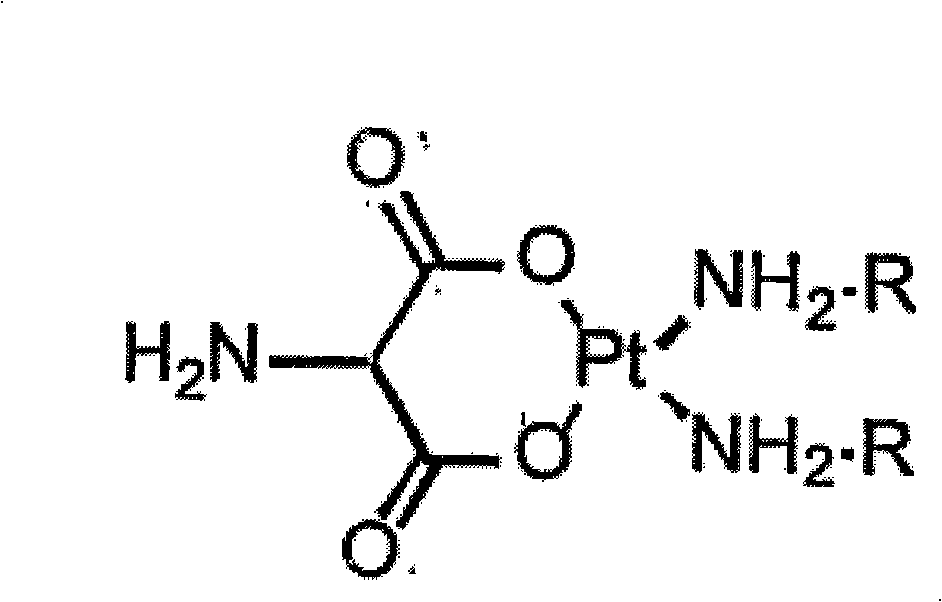
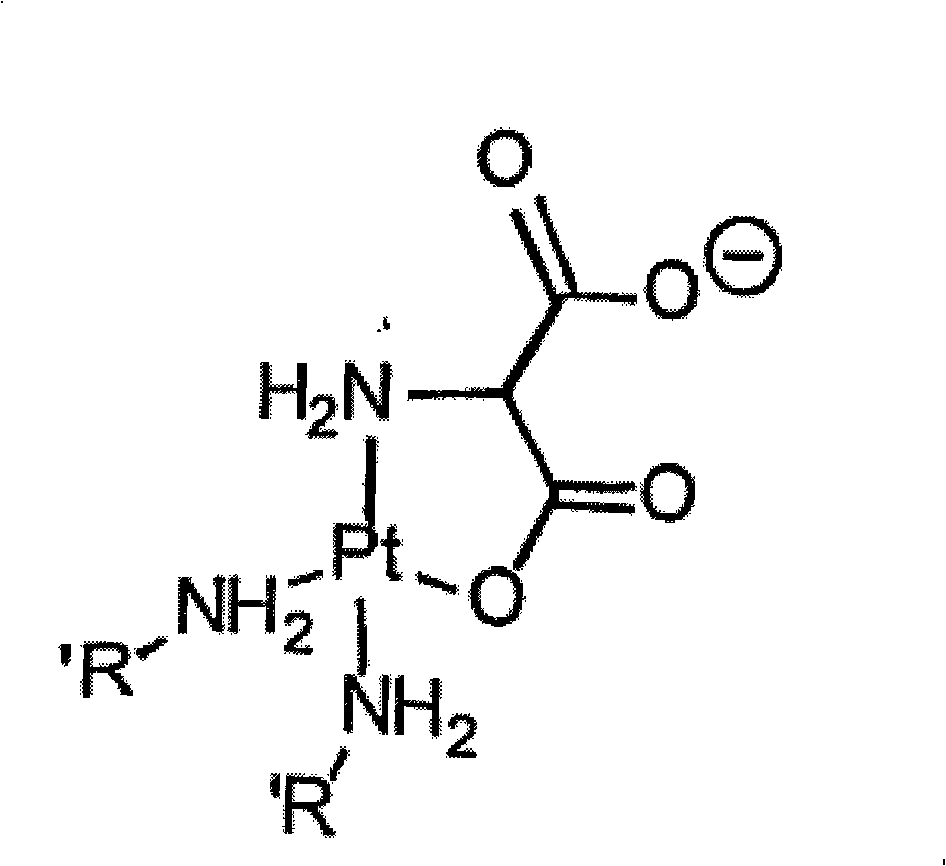
O,O'-amino malonate and N, O-amino malonate platinum complex compound
A technology of aminomalonate and aminomalonic acid, which is applied in the field of O, O'-aminomalonate and N, O-aminomalonate platinum complexes, and can solve problems that have not been evaluated clinically. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0192] chemical reagent
[0193] Sigma-Aldrich of the United States provides cisplatin, pyrimidine, ethanol, ethyl acetate, diethyl ether, diethylaminomalonate, diethyl N-acetylmalonate, silver nitrate, sodium hydroxide, 1R, 2R- Diaminocyclohexane, polyglutamic acid sodium salt, potassium iodide and PBS buffer. Solvents are of HPLC grade, while reagents are of ACS grade or higher. BioRad Laboratories offers ion exchange media, AG 501-X8(D)H + ,HO - Dosage Form, AG 50W-X8H + , and Chelex100 biotech grade. Grade 1 water was obtained using the Milli-Q water system. K 2 PtCl 4 Provided by All-Chemie., Mt. Pleasant, SC. Acid Filtration Slurry No. 289 was from Schleicher and Schuell. Poly(HPMA)-GFLG-ONp, poly(HPMA)-GFLG-Ama-diEt (45kDa), and poly(HPMA)-GFLG-Ama-diEt (351kDa) were synthesized by Polymer Laboratories, Shropshire, UK. Amino acid analysis as well as MALDI-TOF-MS were performed by Gaithersburg, MD, Peptide Technologies Corp.
[0194] Instruments and Equipment ...
example 1
[0208] Example 1: Preparation of p(HPMA)-GFLG-Ama-diEt, about 25kDa
[0209] A dry 1 L round bottom flask with a magnetic stir bar was capped with a septum and cooled in a vacuum. Once cooled, nitrogen was added, the septum was opened, and 29.79 g (140.8 mmol) of diethylaminomalonate was added. The septum was removed and 800 ml of anhydropyrimidine was cannulated into the flask. Add 50g poly(HPMA)-GFLG-ONp (compound 1, US5,965,118 figure 1 A) one-third of the amount. When nearly dissolved, another third of the amount of ONp-polymer was added. When the second third of the volume is nearly dissolved, the final third of the ONp-polymer is added.
[0210] The free and total p-nitrophenols in the reaction were monitored by HPLC, using C18 column, pH4.5MeCN mobile phase, and 316nm ultraviolet detection. After basic hydrolysis (pH 12, 5 minutes), the quantitative solution was analyzed for free and total p-nitrophenol. After stirring for 20-24 hours at 23°C, the reaction was sub...
example 2
[0213] Example 2: the preparation of cis-cisplatin (II) nitrate
[0214] At room temperature, a suspension consisting of cisplatin (8.996g, 29.98mmol), silver nitrate (9.959g, 58.62mmol), 3-5 drops of 5% nitric acid and 190ml of water was placed in a low transmittance bottle and sealed with foil , stirred overnight, then heated at 60-65°C for 3.5 hours. After cooling below 30°C, the mixture was filtered through a 0.22 μm filter to obtain a clear solution at pH 2. Platinum and Silver Elemental Analysis (ICP-OES), typically platinum in the range of 15,000-25,000 ppm and silver in the range of 4-14 ppm. Each formulation was analyzed for Pt, and was heated at 55°C for 5 minutes prior to use and then cooled to room temperature.
[0215] cis-cisplatin(II) nitrate di- 15 N isotope preparation shows that at -1582ppm 195 Pt NMR three times, close to the -1580ppm literature value reported by Appleton et al. in 1989.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com