Hydroxycamptothecin sustained-release microsphere and preparation method thereof
A technology of hydroxycamptothecin and slow-release microspheres, which is applied in the direction of microcapsules, pharmaceutical formulas, and medical preparations containing active ingredients, etc. It can solve the problems of poor system reproducibility, low production efficiency, and large particle size. Achieve the effect of low cost, small dosage and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
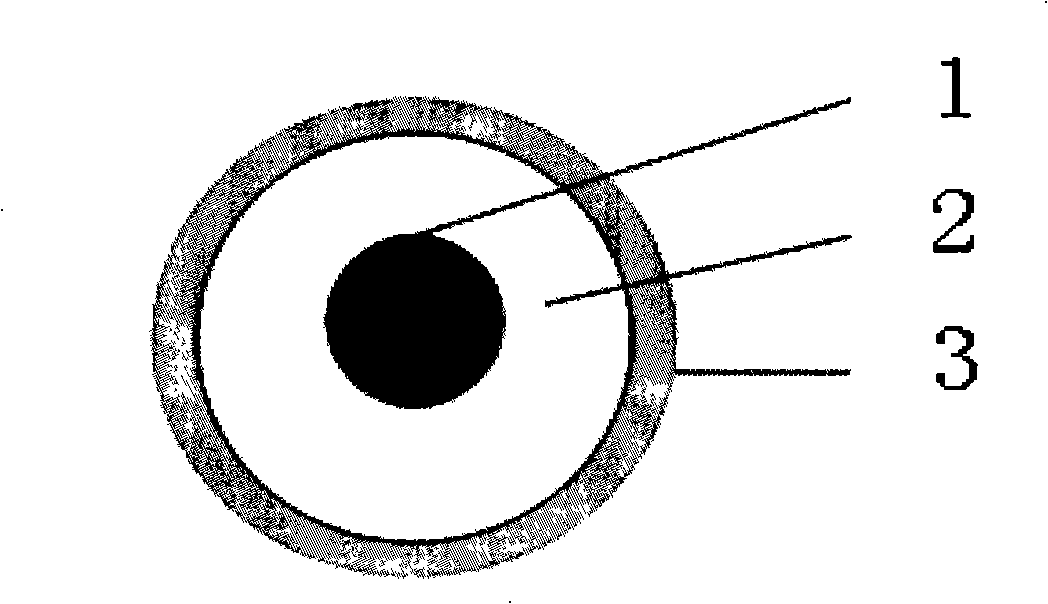
[0025] Embodiment 1: take by weighing 30mg of HCPT crude drug, polylactic acid (PLA) 300mg that molecular weight is 5000, put in beaker with 30ml DMF and CH 2 Cl 2 The mixed solution is dissolved; the mixed aqueous solution of 5% polyvinyl alcohol (PVA) and 1% sodium dodecyl sulfate (SDS) is used as the continuous phase, and the drug-loaded particles are prepared by a membrane emulsifier; the obtained emulsion is centrifuged at a speed of 2000r / min, Collect the precipitate and lyophilize to obtain the drug-loaded microspheres before modification; then weigh 10 mg of the drug-loaded microspheres. Add 10 ml of 0.5% N-succinyl chitosan in PBS solution during stirring, coat for 1 hour, collect the precipitate after centrifugation, wash with water for 3 times, place in a 40°C oven and vacuum-dry to obtain a powder preparation. figure 1 Provide the composition schematic diagram of the hydroxycamptothecin microsphere of the embodiment of the present invention, carrier 2 is polylacti...
Embodiment 2
[0026] Embodiment 2: take by weighing 20mg of HCPT crude drug, polylactic acid (PLA) 400mg that molecular weight is 10000, put in beaker with 40ml DMF and CH 2 Cl 2 The mixed solution is dissolved; the mixed aqueous solution of 0.5% polyvinyl alcohol (PVA) and 0.5% sodium dodecyl sulfate (SDS) is used as the continuous phase, and the drug-loaded particles are prepared by a membrane emulsifier; the obtained emulsion is centrifuged at a speed of 1800r / min, Collect the precipitate and lyophilize to obtain the drug-loaded microspheres before modification; then weigh 50 mg of the drug-loaded microspheres. Add 10 ml of 0.5% N-succinyl-O-carboxymethyl chitosan in PBS solution during stirring, coat for 1 hour, collect the precipitate after centrifugation, wash with water for 3 times, and vacuum-dry in an oven at 45°C to obtain a powder preparation.
Embodiment 3
[0027] Embodiment 3: take by weighing 20mg of HCPT crude drug, polylactic acid (PLA) 300mg that molecular weight is 15000, put in beaker with 40ml DMF and CH 2 Cl 2 The mixed solution is dissolved; the mixed aqueous solution of 1% polyvinyl alcohol (PVA) and 0.5% sodium dodecyl sulfate (SDS) is used as the continuous phase, and the drug-loaded particles are prepared by a membrane emulsifier; the obtained emulsion is centrifuged at a speed of 2500r / min, Collect the precipitate and lyophilize to obtain the drug-loaded microspheres before modification; then weigh 30 mg of the drug-loaded microspheres. Add 20 ml of 0.2% N-lactose acylated succinyl chitosan in PBS solution during stirring, coat for 1 hour, collect the precipitate after centrifugation, wash with water for 3 times, place in a 50°C oven and vacuum-dry to obtain a powder preparation.
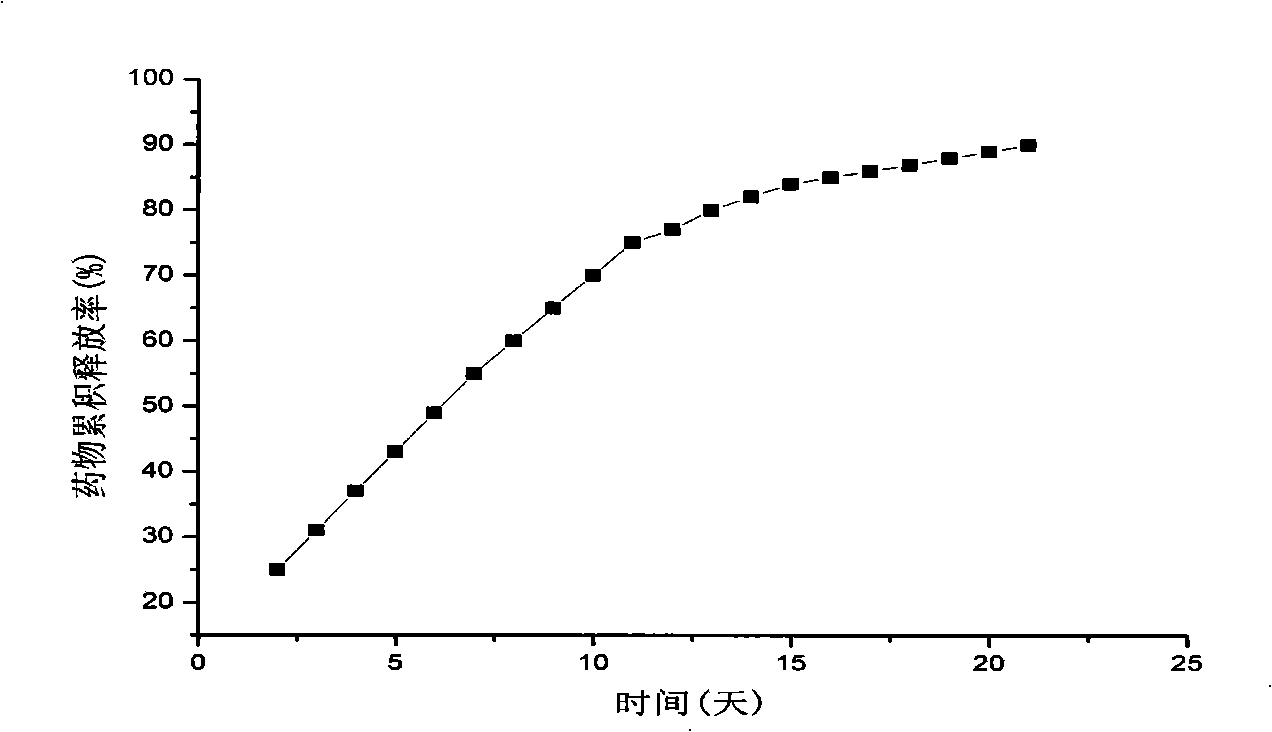
[0028] The drug in vitro cumulative release curve of embodiment 3 is shown in image 3 ,from image 3 It can be seen that the releas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com