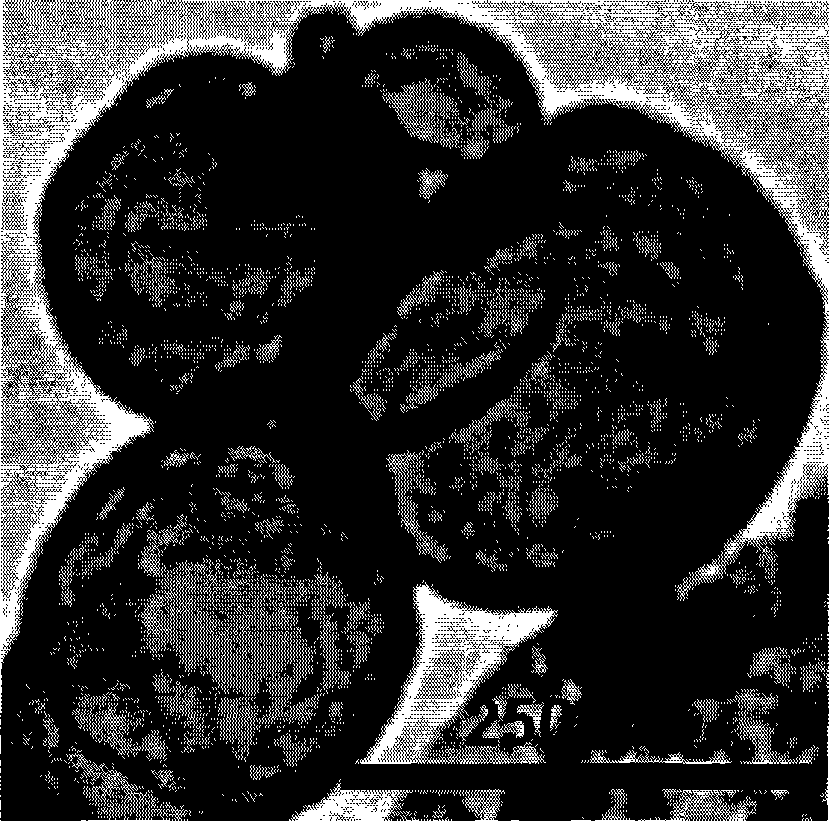Preparation method of liposome embedded quantum dots silicon dioxide microspheres and products thereof
A silicon dioxide and liposome technology, applied in the preparation of microspheres, preparations for in vivo tests, chemical instruments and methods, etc., can solve the problem of only a small amount of quantum dots can be embedded, and overcome the uneven particle size distribution , Improve the intensity of light signal, reduce the effect of toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of silica nanospheres with liposome-embedded quantum dots:
[0050] The preparation of step A, lipid film:
[0051] Accurately weigh soybean phospholipids and cholesterol (mass ratio is 3:2), dissolve phospholipids and cholesterol in a pear-shaped flask with chloroform, rotate to evaporate, evaporate chloroform to make phospholipids and cholesterol form a lipid film on the wall of the flask, blow with nitrogen Dry the film to remove residual chloroform;
[0052] The preparation of step B, liposome:
[0053] Red fluorescent CdTe / CdS quantum dot aqueous solution (basic pH=10) was added to the lipid film, soaked overnight under nitrogen protection, and then shaken for about 1 hour to obtain a liposome suspension embedding CdTe / CdS. In the suspension, the appearance is turbid due to the large liposome particles;
[0054] Step C, embedding of silica:
[0055] (1) Take part of the liposome suspension of CdTe / CdS, filter it with ordinary qualitative filter pape...
Embodiment 2
[0059] Preparation of silica microspheres embedded with quantum dots in water-soluble drug liposomes:
[0060] The preparation of step A, lipid film:
[0061] Accurately weigh soybean phospholipids and cholesterol (mass ratio is 3:2), dissolve phospholipids and cholesterol in a plow flask with chloroform, rotate to evaporate, evaporate chloroform to make phospholipids and cholesterol form a lipid film on the wall of the flask, blow with nitrogen Dry the film to remove residual chloroform;
[0062] The preparation of step B, water-soluble drug liposome:
[0063] Add CdTe / CdS quantum dot aqueous solution and doxorubicin (water-soluble drug) to the lipid film, soak overnight under nitrogen protection, and shake for about 1 hour to obtain a water-soluble drug liposome mixture embedding CdTe / CdS quantum dots and drug. suspension;
[0064] Step C, embedding of silica:
[0065] Use ordinary qualitative filter paper to filter to remove large liposome aggregates, then directly add ...
Embodiment 3
[0068] Preparation of silica microspheres with lipid-soluble drug liposomes embedding quantum dots:
[0069] Step A, preparation of fat-soluble drug lipid film:
[0070] Accurately weigh soybean phospholipids and cholesterol (mass ratio is 3:2) and paclitaxel (fat-soluble drug), dissolve phospholipids and cholesterol in a plow flask with chloroform, add paclitaxel, rotary evaporate, and evaporate chloroform to make phospholipids, cholesterol and Paclitaxel forms a fat-soluble drug lipid film on the wall of the flask, and nitrogen blows the film to remove residual chloroform;
[0071] The preparation of step B, fat-soluble drug liposome:
[0072] Add CdTe / CdS quantum dot aqueous solution to the lipid film, soak overnight under nitrogen protection, and then shake for about 1 hour to obtain a fat-soluble drug liposome suspension embedding CdTe / CdS quantum dots and drugs;
[0073] Step C, embedding of silica:
[0074] Use ordinary qualitative filter paper to filter to remove la...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com