No-Pt catalyst for fuel cell, preparation method and use thereof
A fuel cell and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of Pt catalyst "poisoning, fuel penetration, performance gap from commercial applications, etc., to achieve high catalytic activity, Good stability and durability, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 0.0183g H 3 Ir(CN) 6 , 0.04834g Co(OCOCH 3 ) 2and 0.025g Vulcan XC-72R toner, placed in an agate mortar. Add 10ml of absolute ethanol and grind thoroughly until the ethanol evaporates completely. The mixture in the agate mortar was transferred to a clean glass beaker, and placed in a vacuum oven at 40 °C for 1 hr to dry under vacuum. Afterwards, the dried mixture was placed in a quartz boat, and calcined at 800° C. for 2 hr in an Ar atmosphere. Obtain the required IrCo / C catalyst.
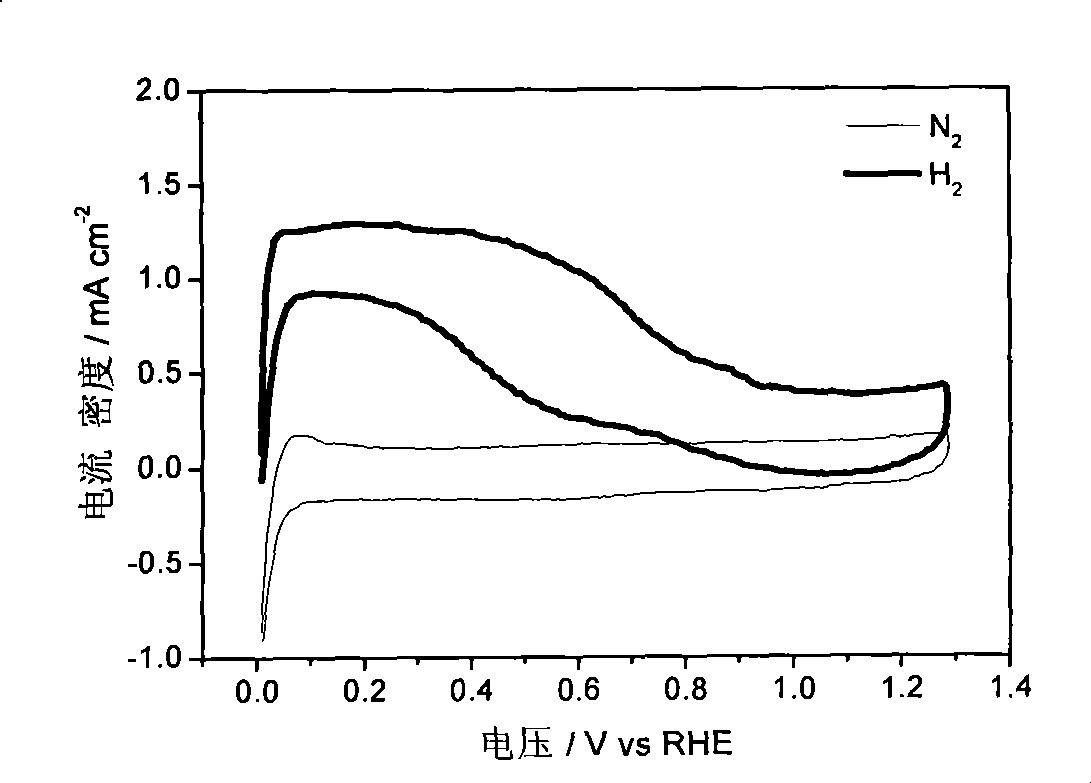
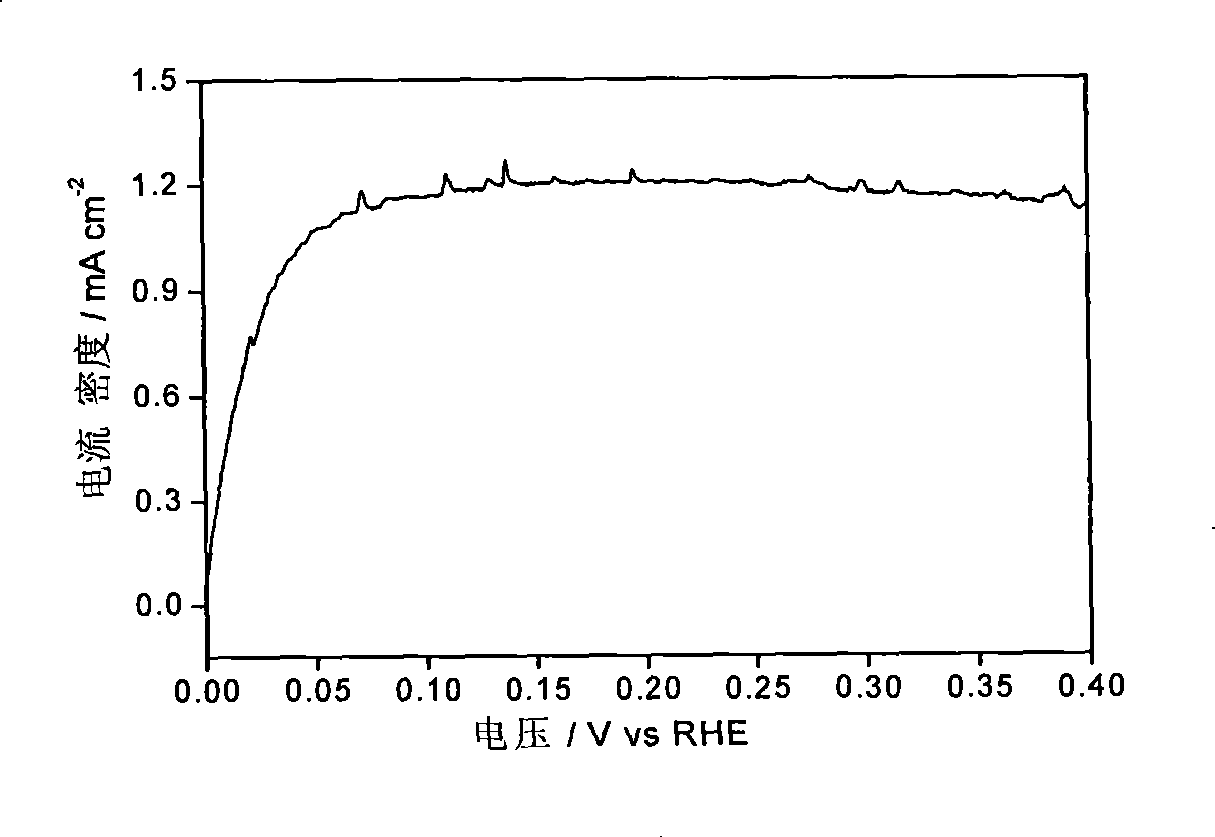
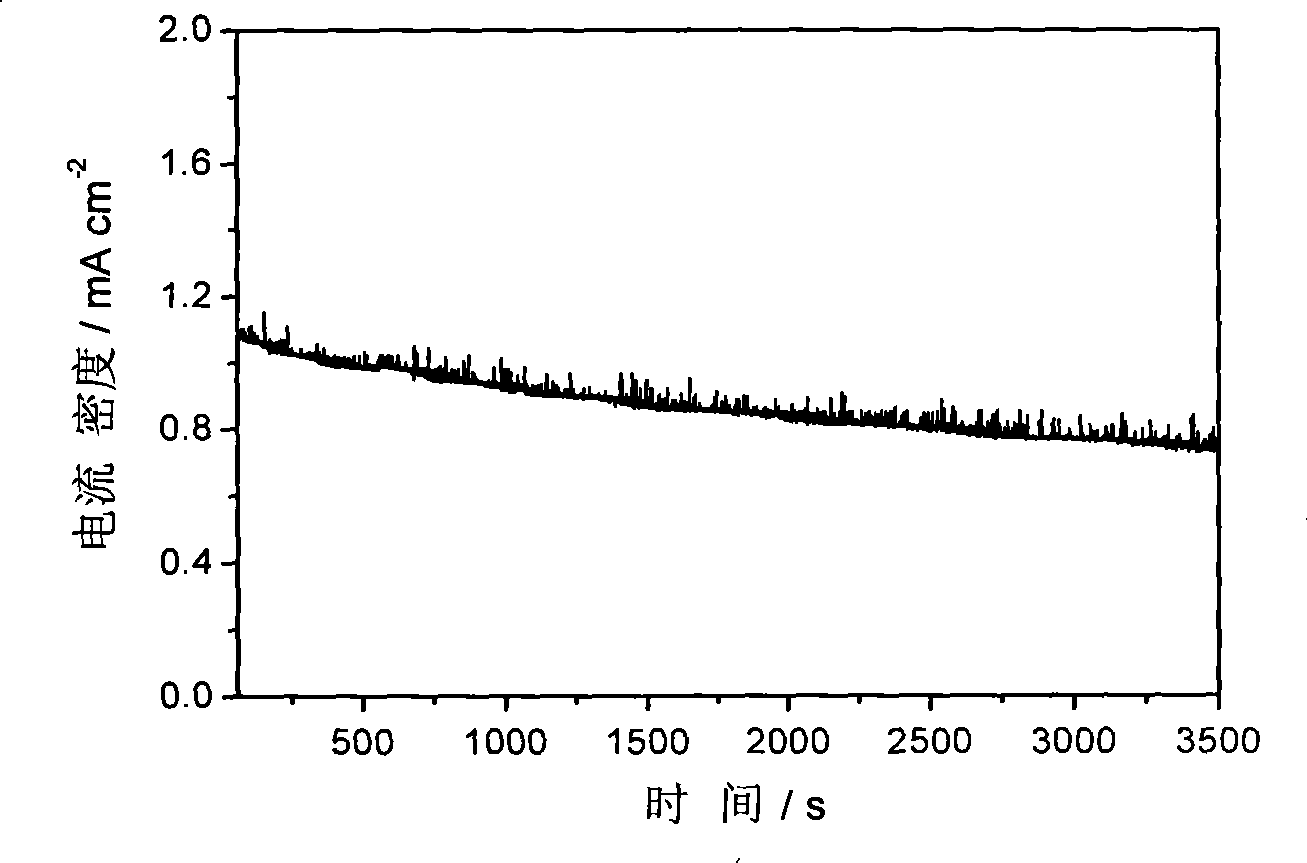
[0031] Figure 1-Figure 3 The electrocatalytic properties of the IrCo / C catalysts are listed. The electrochemical performance tests of IrCo / C catalysts were carried out in a traditional three-electrode system. Electrolyte is 0.1M HClO 4 , the working electrode is a glassy carbon (Glassy Carbon, GC) electrode loaded with IrCo / C catalyst, the reference electrode is a standard hydrogen electrode, and the counter electrode is a Pt foil electrode. First, 1 mg of IrCo / C catalyst wa...
Embodiment 2
[0034] Weigh 0.183g H 3 Ir(CN) 6 , 0.521g V(OCOCH 3 ) 2 Put 0.25g of Vulcan XC-72R carbon powder in an agate mortar, add 10ml of absolute ethanol, fully grind for 30min, and mix until the ethanol evaporates completely.
[0035] The mixture in the agate mortar was transferred to a clean glass beaker, and then placed in a vacuum oven at 40° C. for 1 hr to dry under vacuum. Afterwards, the dried mixture was placed in a quartz boat, in an Ar atmosphere, the temperature was controlled to rise at 20° C. / min, and then calcined at 400° C. for 2 hr. Obtain the required IrV / C catalyst.
[0036] The electrochemical testing method of IrV / C catalyst is similar to embodiment 1. From Figure 4 It can be found that the catalyst exhibits high catalytic activity.
Embodiment 3
[0038] Weigh 0.183g H 3 Ir(CN) 6 , 0.472g Ni(OCOCH 3 ) 2 Put 0.25g of carbon black in an agate mortar, add 10ml of absolute ethanol, and fully grind for 30min. The mixture in the agate mortar was transferred to a clean glass beaker, and then vacuum dried in a vacuum oven at 60°C for 1 hr. Afterwards, the dried mixture was placed in a quartz boat, in an Ar atmosphere, the temperature was controlled to rise at 20° C. / min, and then calcined at 400° C. for 2 hr. Obtain the required IrNi / C catalyst.
[0039] The electrochemical testing method of IrNi / C catalyst is similar to embodiment 1. From Figure 5 It can be found that the catalyst exhibits high catalytic activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com