Method for preparing telbivudine
A technology of telbivudine and thymidine, applied in sugar derivatives, organic chemistry, etc., can solve the problems of unfavorable environmental protection and safe production, low economic and social public benefits, and low comprehensive benefits, and achieve effective It is beneficial to environmental protection and safe production, reduces synthesis time and production cost, and improves comprehensive benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
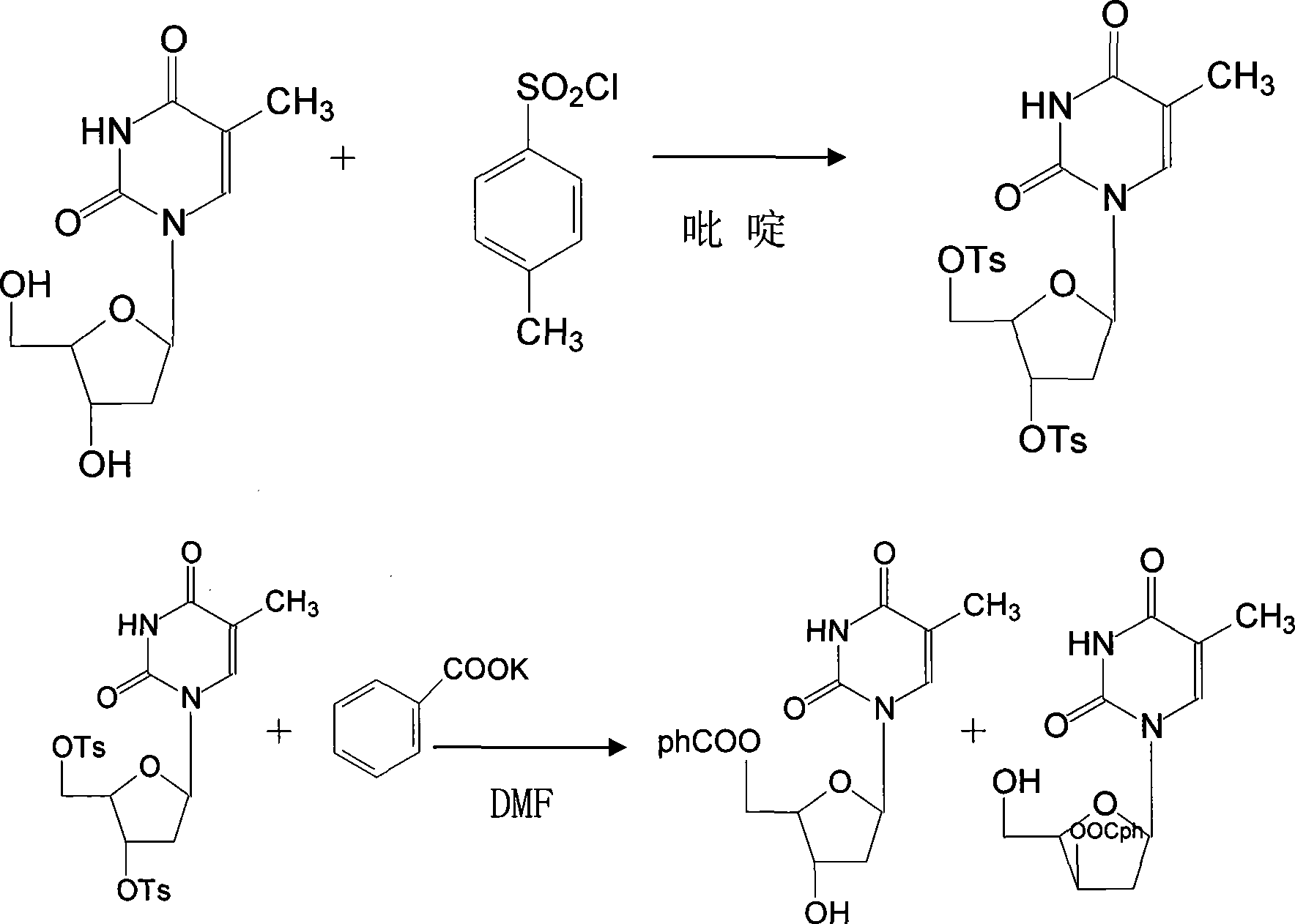
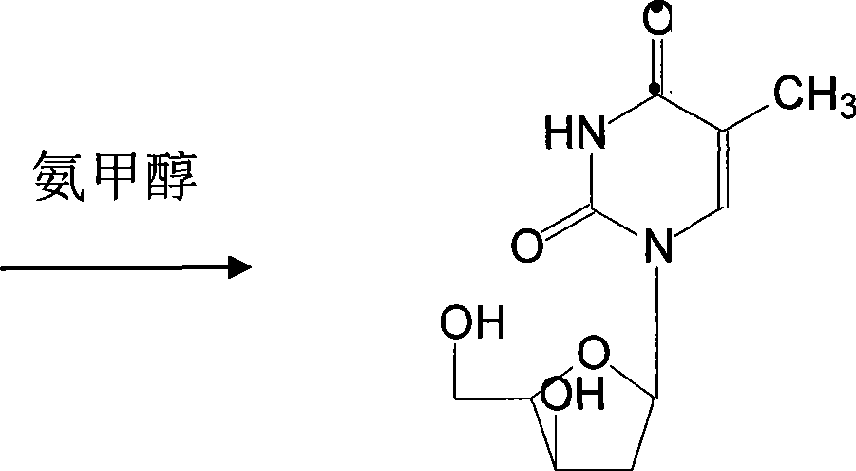
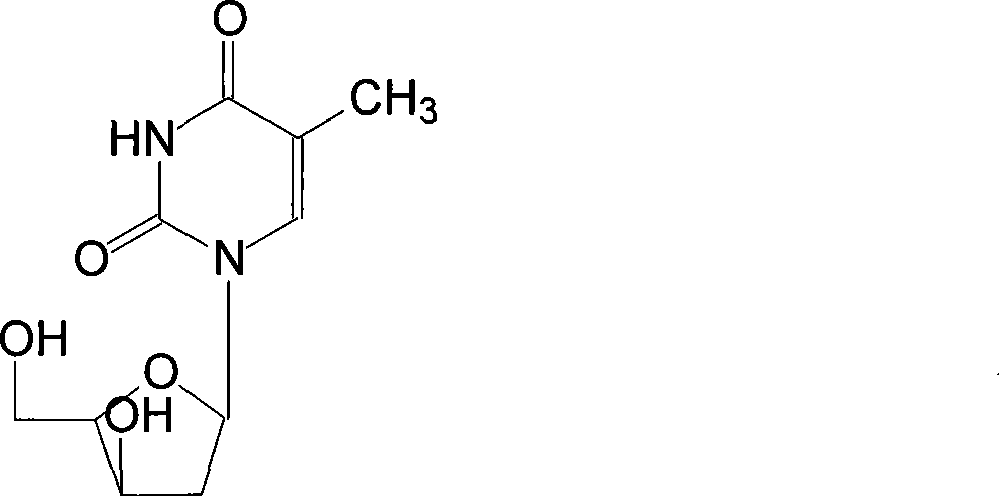
[0024] The preparation of telbivudine uses 2'-deoxy-β-D-thymidine as the starting material, uses anhydrous pyridine as the solvent, and reacts with p-toluenesulfonyl chloride at a temperature of -5-0°C After 8-15 hours, 3,5-di-O-p-toluenesulfonyl-β-D-thymidine was generated, then added pyridine aqueous solution, centrifuged to remove the mother liquor, and dried to obtain a white crystal powder with a yield of 85 -93%; Add DMF to the above powder as a solution, that is, dimethylformamide solution, mix well, and at a temperature of 70-100°C, 3,5-di-O-tosyl-β-D-thymus Pyrimidine nucleoside reacts with potassium benzoate for 3-9 hours, undergoes transformation, removes p-toluenesulfonyl group, generates 3,5-di-O-benzoyl-β-L-thymidine, evaporates and concentrates, completely DMF was removed, and the yield was 78-86%; ammonia methanol was added to the above solution to hydrolyze 3,5-di-O-benzoyl-β-L-thymidine to obtain the target 2′-deoxy- β-L-thymidine, then evaporated and concen...
Embodiment 2
[0026] In the preparation method of telbivudine, 2′-deoxy-β-D-thymidine reacts with toluenesulfonyl chloride, the temperature is controlled at -4-0°C, and the time is 9-14 hours; 3,5- The reaction temperature of di-O-p-toluenesulfonyl-β-D-thymidine and potassium benzoate is controlled at 80-95° C. and the time is 4-8 hours. Other process steps and conditions are the same as in Example 1.
Embodiment 3
[0028] In the preparation method of telbivudine, 2'-deoxy-β-D-thymidine reacts with toluenesulfonyl chloride, the temperature is controlled at -4-1°C, and the time is 10-13 hours; 3,5 - Di-O-p-toluenesulfonyl-β-D-thymidine reacts with potassium benzoate at a controlled temperature of 85-90°C for 5-7 hours; 3,5-di-O-benzoyl- Methanol was used for recrystallization in the hydrolysis reaction of β-L-thymidine and ammonia methanol, and other process steps and conditions were the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com