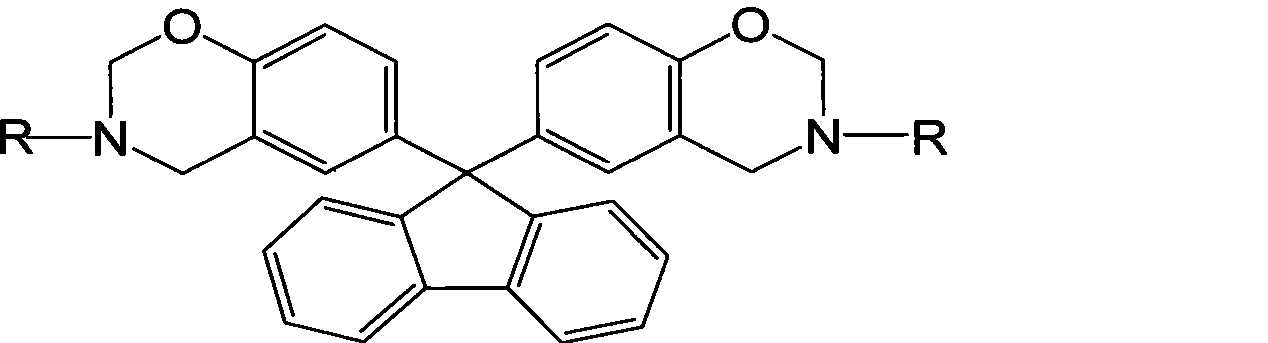
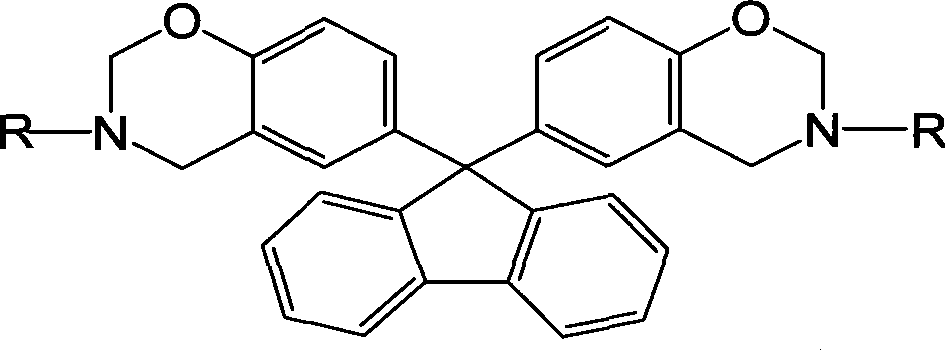
Fluorenyl bi-benzoxazine monomer and method of preparing the same
A benzoxazine and fluorene-based double technology is applied in the field of organic polymer material monomers to achieve the effects of improving rigidity, improving humidity and heat resistance, and reducing water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 250mL three-necked flask equipped with a stirrer, a condenser, and a thermometer, add 6.5g of formaldehyde solution, stir at room temperature for 30 minutes, slowly add 3.7g of aniline dropwise, stir and react at room temperature for 2 hours, then raise the temperature to 90°C, add 33% bismuth The compound solvent solution of phenol fluorene (mass fraction) is 21g, the mass ratio of ethanol and dioxane in the compound solvent is 1:1, and the reaction is 6h. After the reaction, dissolve with ether, wash with alkali, wash with water, separate liquid, evaporate the organic solvent under reduced pressure, and dry to obtain the light yellow benzoxazine monomer, and the product yield can reach 74.92%.
Embodiment 2
[0021] In a 250mL three-necked flask equipped with a stirrer, a condenser, and a thermometer, add 1.62g of formaldehyde solution, stir at room temperature for 30 minutes, slowly add 2.15g of o-toluidine dropwise, stir and react at room temperature for 2 hours, raise the temperature to 90°C, and add 33 % bisphenol fluorene (mass fraction) compound solvent solution 5.3g, the mass ratio of ethanol and dioxane in the compound solvent is 3:1, react for 6h. After the reaction, dissolve with ether, wash with alkali, wash with water, separate liquid, evaporate the organic solvent under reduced pressure, and dry to obtain light yellow benzoxazine monomer, and the product yield can reach 81.68%.
Embodiment 3
[0023] In a 250mL three-necked flask equipped with a stirrer, condenser, and thermometer, add 6.5g of formaldehyde solution, stir at 5°C for 30min, slowly add 2.93g of butylamine dropwise, continue stirring for 2h, then raise the temperature to 95°C, add 33 % bisphenol fluorene (mass fraction) in toluene solution 21g, reacted for 4.5h. After the reaction is completed, dissolve in chloroform, wash with alkali, wash with water, separate liquid, evaporate the organic solvent under reduced pressure, and dry to obtain a light yellow benzoxazine monomer, and the product yield can reach 89.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com