Method for synthesis of 5-bromine-3-sec-butyl-6- methyl uracil
A technology of methyl uracil and a synthesis method is applied in the field of synthesizing 5-bromo-3-sec-butyl-6-methyl uracil and achieves the effects of competitiveness, reduction of reaction time and environmental protection of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
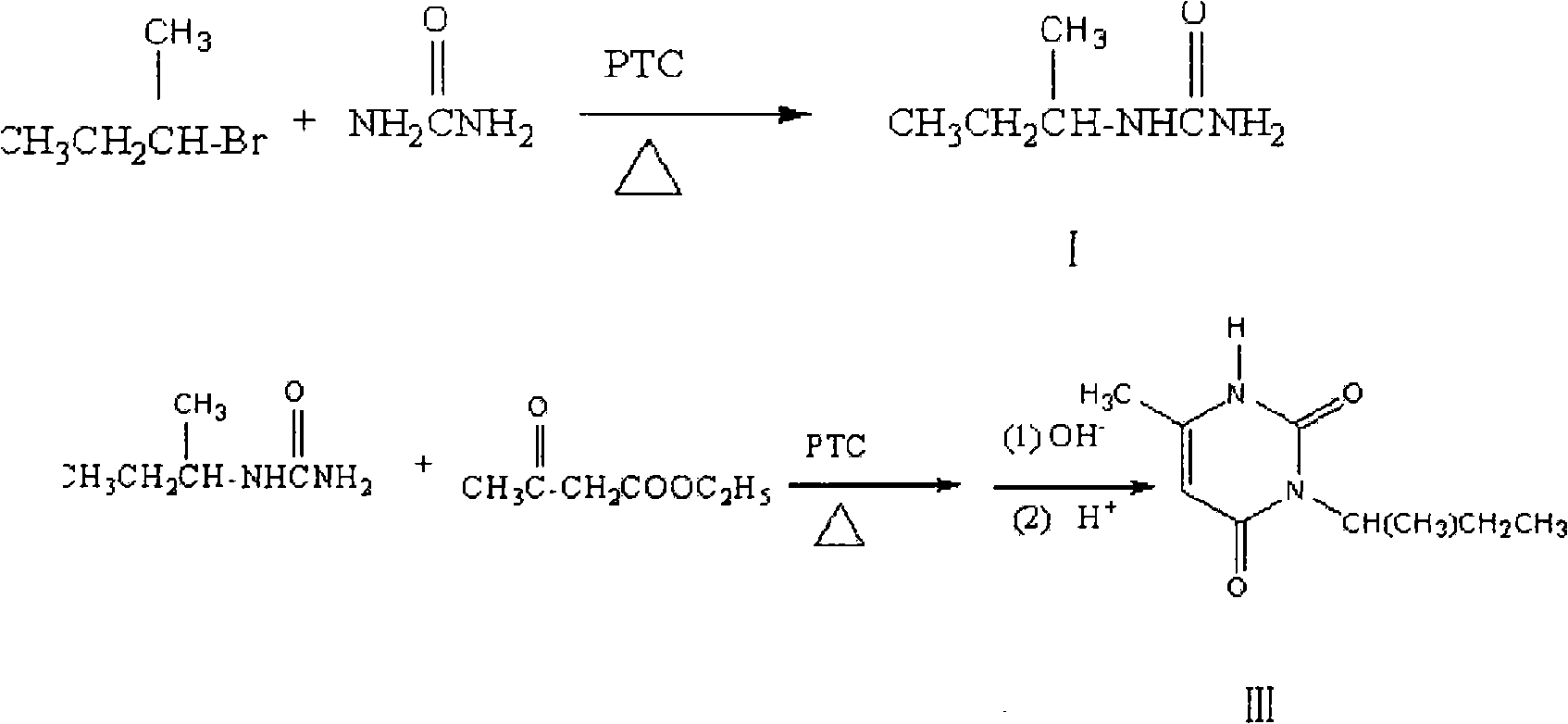
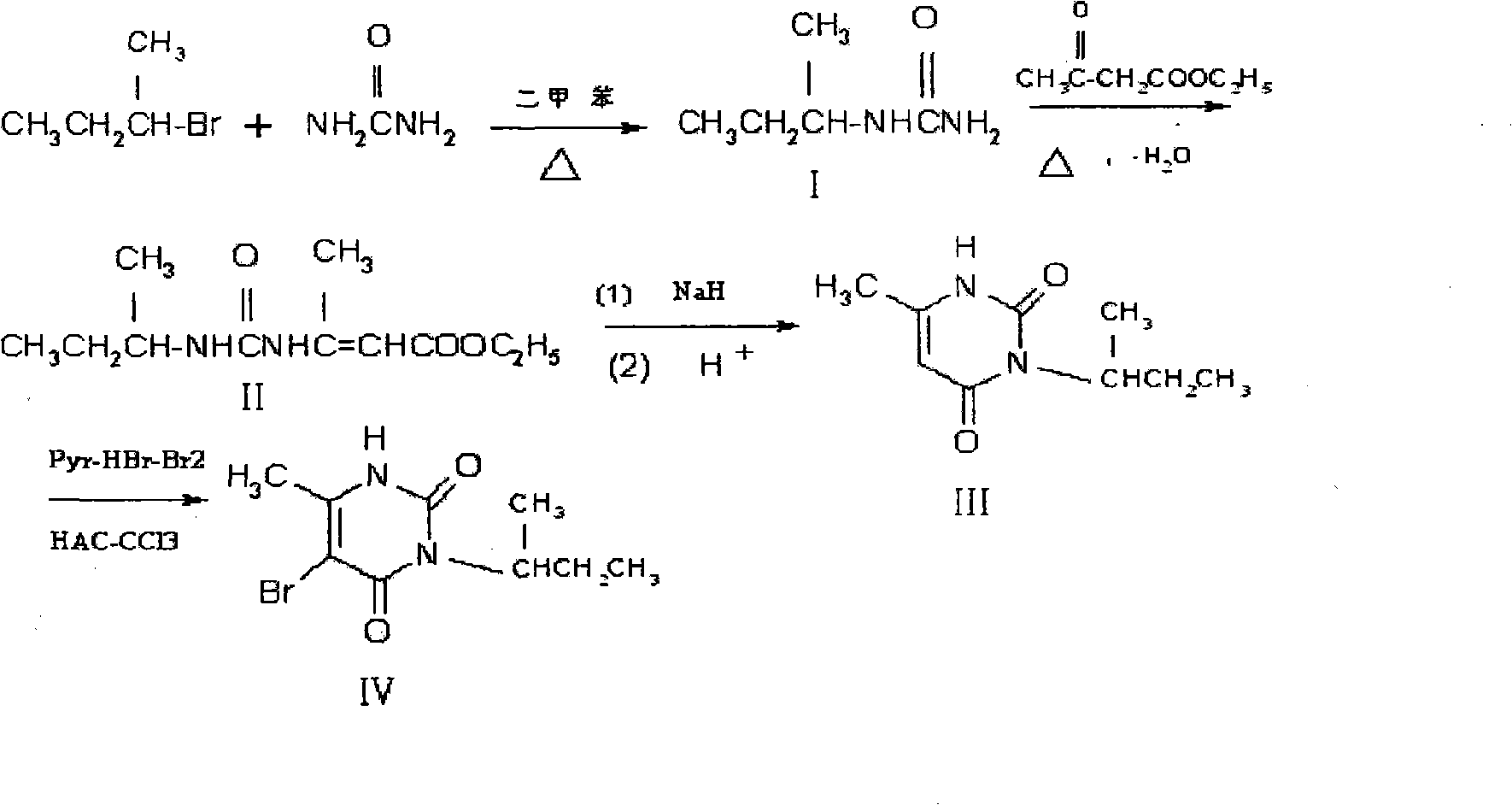
[0043] The synthetic method of embodiment 1. sec-butylurea (I):
[0044] Add 13.7g (0.1mol) of 2-bromobutane, 100ml of polyethylene glycol-400 (PTC), and 8.4g (0.15mol) of urea into a 250ml three-necked reaction flask equipped with a thermometer, a reflux condenser, and electric stirring , under stirring, reflux reaction for 6h, TLC thin-layer chromatography detection raw material point no, indicating the completion of the reaction. Cool to room temperature, filter with suction, wash the liquid with saturated brine (2×10ml) twice, then dry with anhydrous sodium sulfate for 1 h, filter the filtrate with suction, distill under reduced pressure with a rotary evaporator to obtain a solid, and weigh it with ethanol Crystallization to obtain a white product - sec-butylurea 10.90g, yield 94%, melting point: 169 ~ 171 ° C. IR (KBr tablet, υPcm-1) 3453.35 (NH 2 telescopic), 1671.25 (C=0), 2987.28 (-CH 2 ,-CH3 ); 1H NMR (CDCl 3 )δ: 10.81 (S, 2H, -NH 2 ), 10.08(d, 1H, -NH 2 ), 3.95 ...
Embodiment 2
[0045] The synthetic method of embodiment 2.3-sec-butyl-6-methyl-uracil (III):
[0046] The sec-butylurea (I) of 11.6g (0.1mol), Polyethylene Glycol-400 (PTC) 100ml adds in the 250ml four-necked reaction flask that thermometer, reflux condenser, constant pressure dropping funnel, electric stirring are housed, Heat to make it reflux, and then slowly drop 13.0 g (0.1 mol) of ethyl acetoacetate into the reaction flask under stirring, and drop it completely within 30 minutes. Then the reaction was refluxed for 3 h, and the reaction was detected by TLC thin layer chromatography to complete. The product was not separated, directly added 2.4g (0.1mol) of sodium hydride to the reaction solution, and refluxed for 5 hours while stirring. TLC thin layer chromatography detected that the reaction was complete, and adjusted the pH of the reaction solution to 3 with 20% hydrochloric acid, cooled Afterwards, the obtained solid was recrystallized with ethyl acetate-acetone (v:v=3:1) to obtain...
Embodiment 3
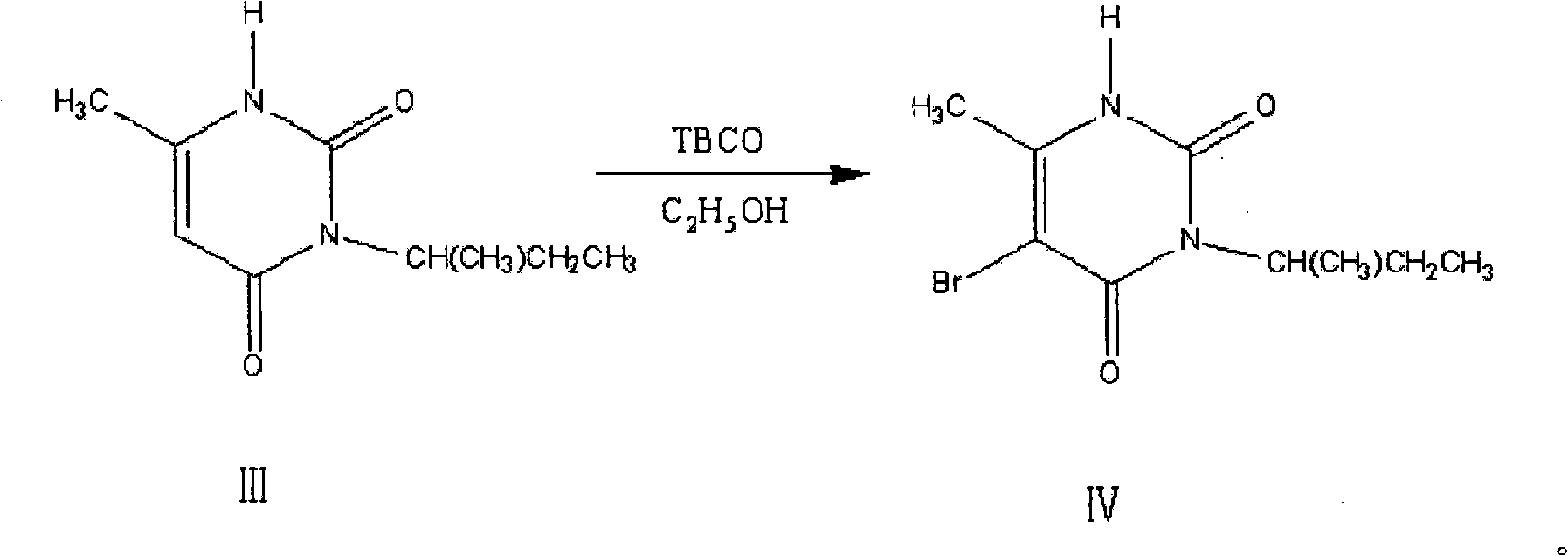
[0047] The synthetic method of embodiment 3.5-bromo-3-sec-butyl-6-methyluracil (IV):
[0048] Add 18.2g (0.1mol) of 3-sec-butyl-6-methyl-uracil (III), 150ml of chloroform and 50ml of glacial acetic acid into a three-necked reaction flask equipped with a thermometer, a reflux condenser, and electric stirring, Stir at room temperature for 30 minutes, then cool down to 0°C in an ice-water bath, add 50ml of brominated reagent tetrabromocyclone (TBCO) and 50ml of absolute ethanol as a solvent, and slowly add it to the reaction flask at 0°C with a dropping funnel. Then react at room temperature for 8h, and TLC thin layer chromatography detects that the reaction is complete. The reactant is distilled under reduced pressure in a rotary evaporator, and the solid obtained is reconstituted with a mixed solvent of acetone-ethyl acetate (v:v=2:3). Crystallized to obtain 21.14g of a white product with a yield of 81% and a melting point of 159-160°C. 1HNMR (400Hz, CDCl3, TMS internal standa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com