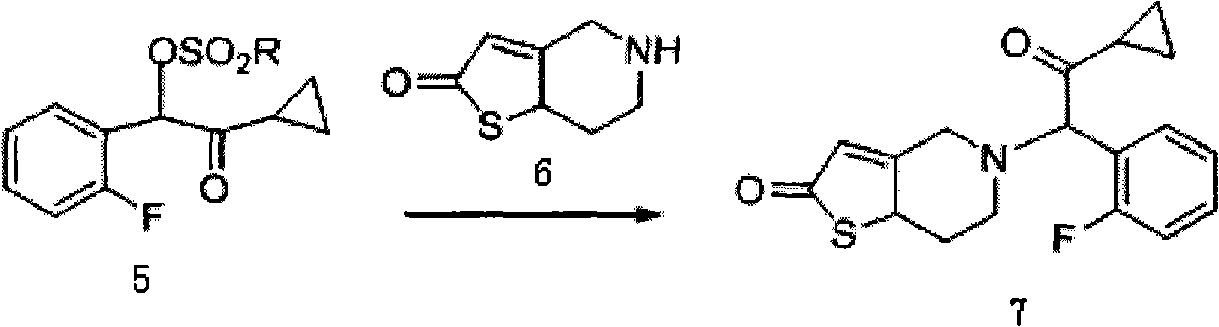
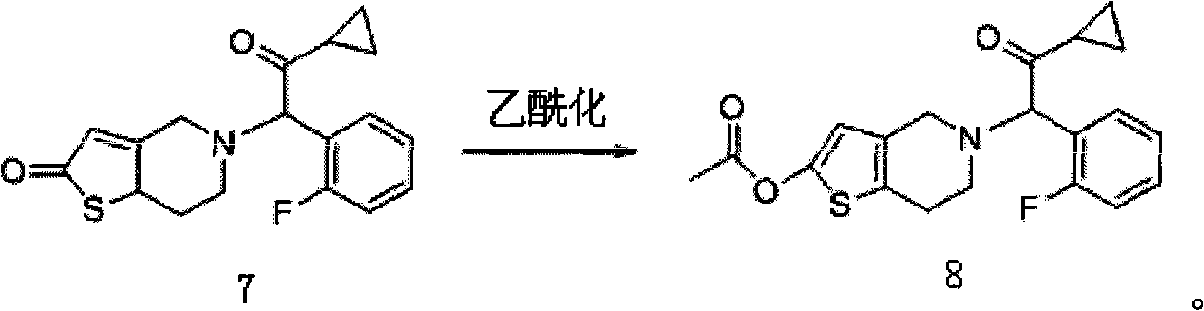
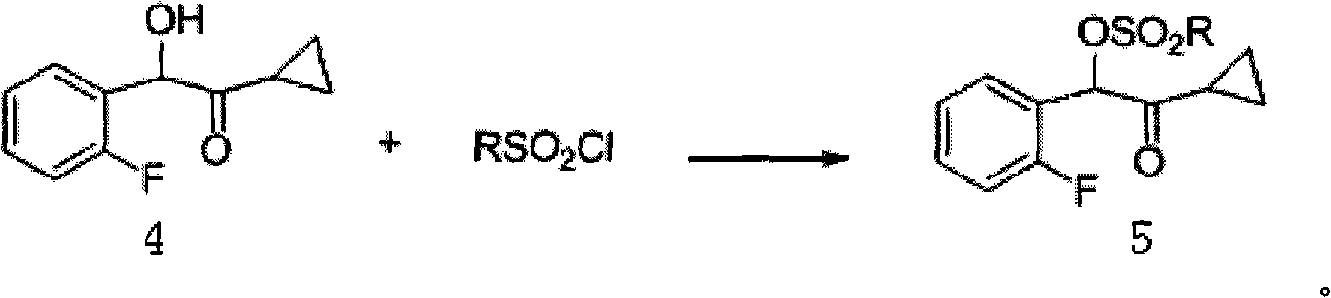Industrial production method for prasugrel
A technology based on compounds and alkaline conditions, which is applied in the field of preparation of antithrombotic drug prasugrel, can solve problems such as high labor protection requirements, toxicity, and large equipment corrosion, and achieve low labor protection requirements, high yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 1-cyclopropyl-2-(2-fluorophenyl) vinyl acetate (compound of formula 3)
[0038] Add 17.8g (0.1mol) of cyclopropyl-2-fluorobenzyl ketone, 120g (1.2mol) of isopropenyl acetate, and 5.7g (0.03mol) of toluene-4-sulfonic acid into the reaction flask, and heat to reflux for reaction. Acetone was continuously distilled off, the reaction was complete, cooled to room temperature, the reaction solution was poured into ice water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and rotary evaporated to obtain 20.9 g of reddish-brown oil, yield: 95.0%.
[0039] 1 HNMR (CDCl 3 )δ: 7.00-7.59 (m, 4H), 6.28 (s, 1H), 2.05-2.23 (m, 3H), 1.90-94 (m, 1H), 0.73-0.79 (m, 2H), 0.62-0.72 ( m, 2H). MS-ESI (m / z): 243.0 (M+Na).
Embodiment 2
[0041] Preparation of 1-cyclopropyl-2-(2-fluorophenyl)-2-hydroxyethanone (compound of formula 4)
[0042] Dissolve 15g (0.068mol) of 1-cyclopropyl-2-(2-fluorophenyl) vinyl acetate in 10mL of dichloromethane, and after cooling to -10°C, add 16.6g (0.082mol) of m-chloro Peroxybenzoic acid, stirred and reacted for 5h, the reaction was complete, extracted with ethyl acetate (80mL*3), the organic phase was washed with saturated brine (50mL*4), anhydrous NaSO 4 After drying and rotary evaporation, 9.8 g of oily substance was obtained, which can be directly used for the next reaction. Yield 75%.
[0043] 1 HNMR (CDCl 3 )δ: 7.10-7.35 (m, 4H), 5.60 (s, 1H), 4.33 (s, 1H), 1.89-1.94 (m, 1H), 0.73-0.79 (m, 2H), 0.62-0.72 (m, 2H). MS-ESI (m / z): 217.0 (M+Na).
Embodiment 3
[0045] Preparation of 1-cyclopropyl-2-(2-fluorophenyl)-2-hydroxyethanone (compound of formula 4)
[0046] Dissolve 5g (0.023mol) of 1-cyclopropyl-2-(2-fluorophenyl) vinyl acetate in 10mL of acetonitrile, add 200mg [((phen) 2 (H 2 O)Fe III ) 2 (μ-O)](ClO 4 ) 4 Catalyst, after cooling to -18°C, add 25mL peroxyacetic acid, stir for 5min; after the reaction is complete, extract with ethyl acetate (10mL*3), wash the organic phase with saturated brine (20mL*4), anhydrous NaSO 4 After drying, the oily substance obtained by rotary evaporation can be directly used for the next reaction. The 3.2g oily substance obtained by rotary evaporation can be directly used for the next reaction. Yield 72%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com