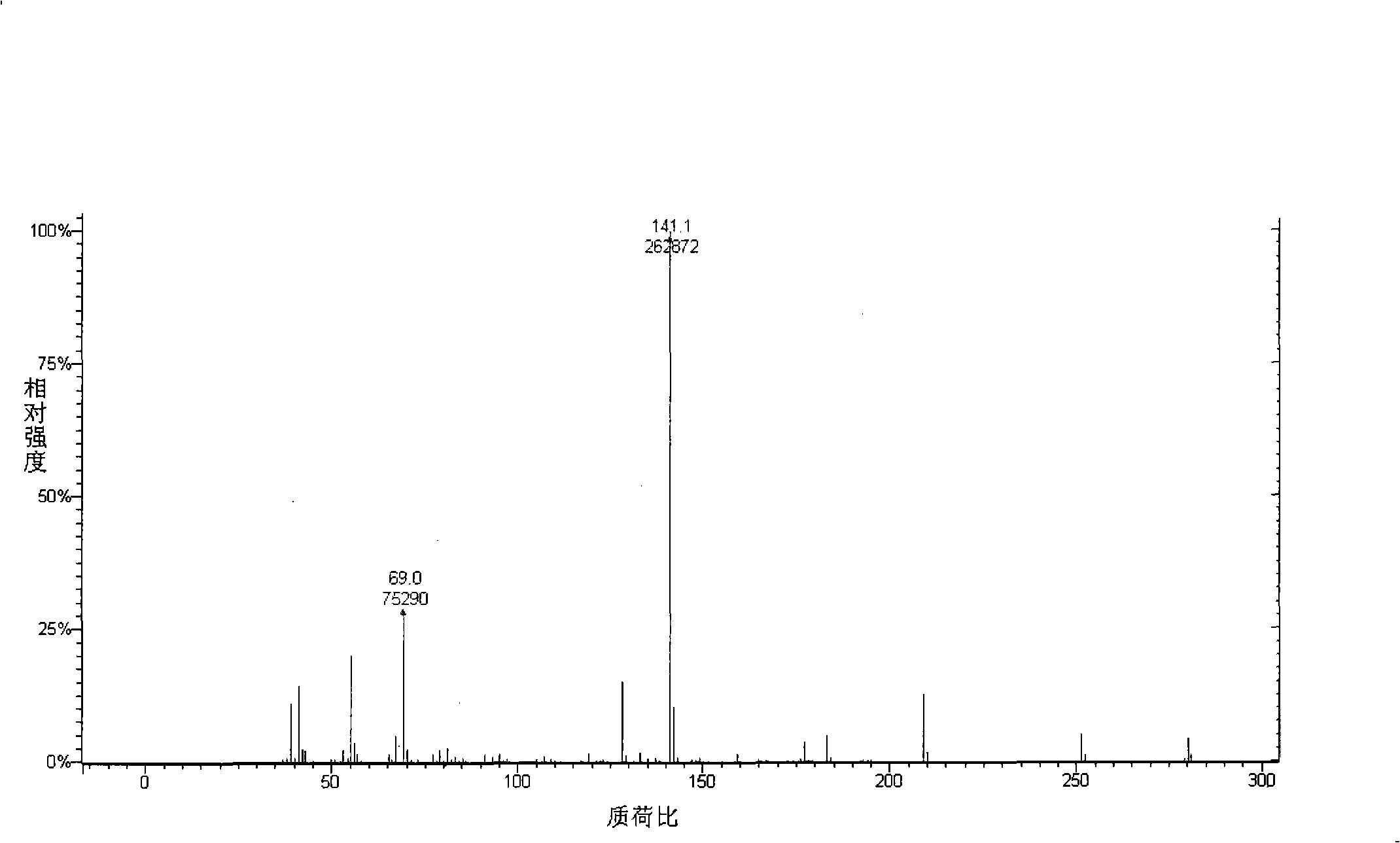
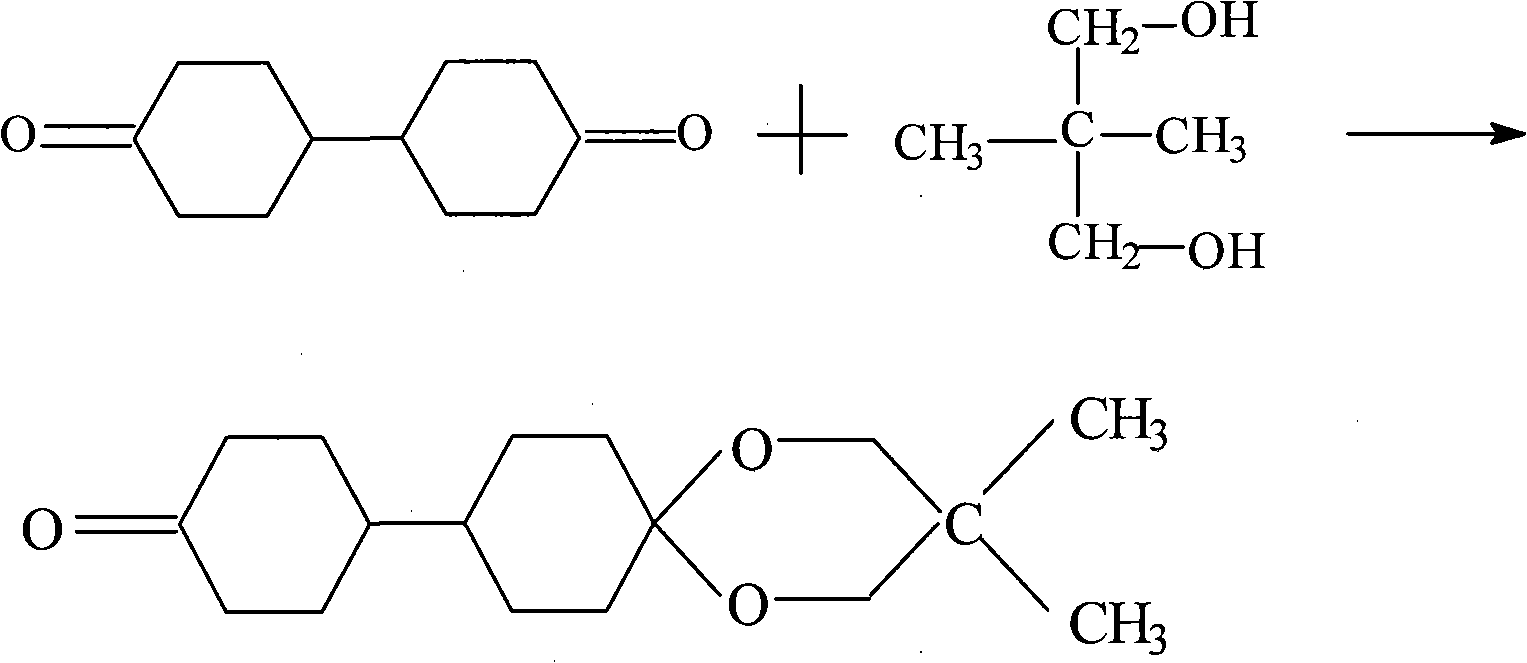
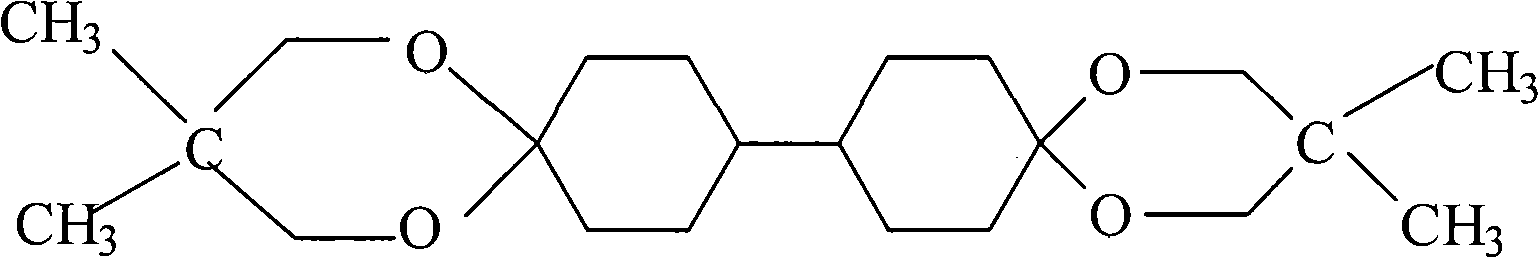
Preparation of bicyclohexyl neopentyl glycol single ketal
A technology of pentylene glycol monoketal and neopentyl glycol, which is applied in the fields of chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., and can solve the problem of troublesome operation process, large environmental pollution, Complicated preparation methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In a 250ml reaction bottle, add 7.76g of dicyclohexyl diketone, 9.95g of neopentyl glycol, that is, the molar ratio of cyclohexyl diketone to neopentyl glycol is 0.7 and 200ml of inert solvent cyclohexane, mix well and add strong 2 g of acidic cation exchange resin was allowed to react at 35° C. for 5.5 h during stirring, and then the resin was removed by filtration to obtain a mixed solution of dicyclohexyl neopentyl glycol monoketal.
[0043] The above-mentioned mixed solution is heated and evaporated until the solvent in the mixed solution is evaporated to dryness, and 500ml of acetone and water mixed solution are added to the obtained solid, and the volume of acetone in the mixed solution of acetone and water accounts for 15% of the total volume. %, the mixed solution was cooled, then filtered, and the filtrate was taken.
[0044] Continue to add 500 mL of water to the above filtrate, then stir for 30 minutes until the solution is uniformly mixed and then filter, an...
Embodiment 2
[0047] In a 2500ml reaction flask, add 98.94g of dicyclohexyl diketone, 59.6g of neopentyl glycol, i.e. the mol ratio of cyclohexyl diketone to neopentyl glycol is 0.9 and 2000ml of inert solvent cyclohexane, mix well and add strong 5.96 g of acidic cation exchange resin was heated to 50° C. for 6 h during stirring, and then the resin was removed by filtration to obtain a mixed solution of dicyclohexyl neopentyl glycol monoketal.
[0048] The above mixed solution was heated and evaporated until the solvent in the mixed solution was evaporated to dryness, and then added to the obtained solid in a mixed solution of 3000ml acetone and water, the volume of acetone in the mixed solution of acetone and water accounted for 20% of the total volume, stirred for 1 hour, then filtered, and the filtrate was taken.
[0049] Continue to add 2000 mL of water to the above filtrate, then stir for 30 minutes until the solution is uniformly mixed and then filter, and recrystallize the obtained s...
Embodiment 3
[0052] In a 250ml reaction bottle, add 11.64g of dicyclohexyl diketone, 6.35g of neopentyl glycol, that is, the molar ratio of cyclohexyl diketone to neopentyl glycol is 1.1 and 200ml of inert solvent cyclohexane, mix well and then add 1.3 g of strongly acidic cation exchange resin was heated to 110° C. during the stirring process, and after reflux and water separation for 3 hours, the resin was removed by filtration to obtain a mixed solution of dicyclohexyl neopentyl glycol monoketal.
[0053] The above-mentioned mixed solution is heated and evaporated until the solvent in the mixed solution is evaporated to dryness, and then added to the obtained solid in a mixed solution of 400ml acetone and water, the volume of acetone in the mixed solution of acetone and water accounts for 1% of the total volume 25%, cooled, and then filtered to get the filtrate.
[0054] Continue to add 300ml of water to the above filtrate, then stir for 30 minutes until the solution is uniformly mixed,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com