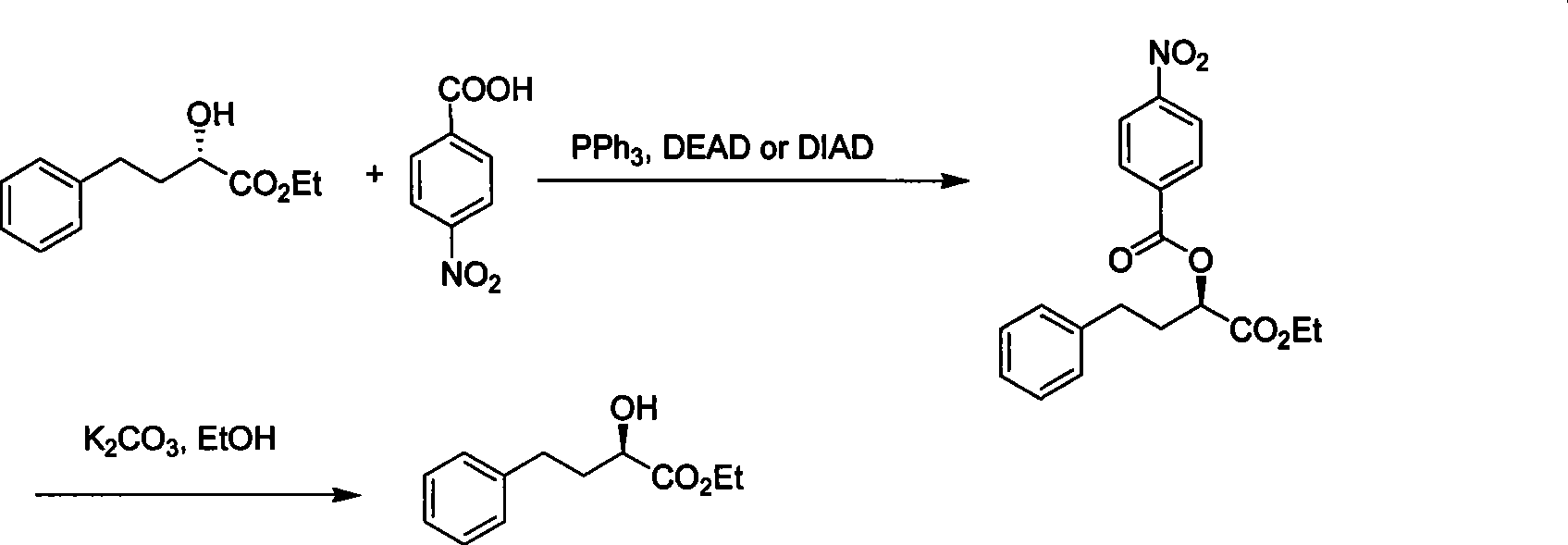
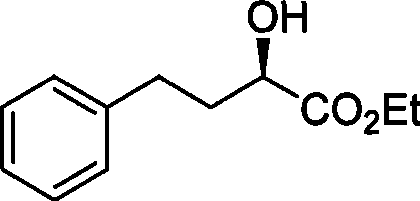
Chemical synthesis method for R-2-hydroxy-4-phenylbutanoate
A technology of chemical synthesis of ethyl phenylbutyrate, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of high atom economy, long reaction process cycle time, poor optical selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 1-liter dry three-necked flask, add ethyl azodicarboxylate (0.12 mol), triphenylphosphine (0.12 mol), 20.8 g of ethyl S-2-hydroxy-4-phenylbutyrate (0.1 mol), p-nitrobenzoic acid (0.11 mol) and 500 milliliters of tetrahydrofuran, mechanically stirred for 5 hours, ended the reaction, then filtered, and the filtrate was concentrated to recycle tetrahydrofuran solvent, followed by recrystallization with ethyl acetate and petroleum ether to obtain 32 grams of R- Esterification intermediate. The intermediate was catalyzed by potassium carbonate (0.25 mole) in 300 milliliters of ethanol to carry out transesterification reaction for 12 hours, then filtered, the filtrate was neutralized with hydrochloric acid, extracted with dichloroethane after recovering the ethanol solvent, and the organic phase was recovered under normal pressure. Ethane was then distilled under reduced pressure, and the fractions at 100-108°C were collected at 40mmHg to obtain 18g of colorless liquid w...
Embodiment 2
[0024] In a 1-liter dry three-necked flask, sequentially add isopropyl azodicarboxylate (0.13 mol), triphenylphosphine (0.13 mol), 20.8 g of S-2-hydroxyl-4-phenylbutyric acid ethyl ester ( 0.1 mole), p-nitrobenzoic acid (0.13 mole) and 500 milliliters of toluene, mechanical stirring reaction 1 hour, finish reaction, filter subsequently, filtrate concentrates and reclaims toluene solvent, then obtains 32.3 grams of R with ethyl acetate and sherwood oil recrystallization - Esterification intermediates. The intermediate was catalyzed by potassium carbonate (0.20 moles) in 300 milliliters of ethanol to carry out transesterification reaction for 15 hours, then filtered, the filtrate was neutralized with hydrochloric acid, extracted with dichloroethane after recovering the ethanol solvent, and the organic phase was recovered under normal pressure. Carry out underpressure distillation after ethane, collect the cut of 100~108 ℃ at 40mmHg, obtain colorless liquid 17.7g, purity 99.0% (H...
Embodiment 3
[0026] In a 1-liter dry three-necked flask, sequentially add isopropyl azodicarboxylate (0.10 mol), triphenylphosphine (0.10 mol), 20.8 grams of S-2-hydroxyl-4-phenylbutyric acid ethyl ester ( 0.1 mole), p-nitrobenzoic acid (0.105 mole) and 500 milliliters of dichloroethane, mechanical stirring reaction 10 hours, finish reaction, filter subsequently, filtrate concentrates and reclaims dichloroethane solvent, then with ethyl acetate and sherwood oil Recrystallization gave 30.3 g of an R-esterified intermediate. The intermediate was catalyzed by potassium carbonate (0.25 mole) in 300 milliliters of ethanol to carry out transesterification reaction for 12 hours, then filtered, the filtrate was neutralized with hydrochloric acid, extracted with dichloroethane after recovering the ethanol solvent, and the organic phase was recovered under normal pressure. Carry out underpressure distillation after ethane, collect the cut of 100~108 ℃ at 40mmHg, obtain colorless liquid 15.5g, purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com