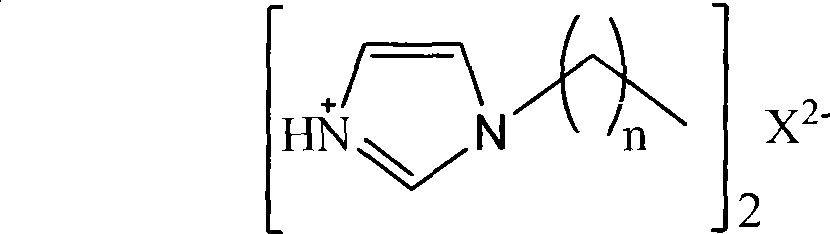
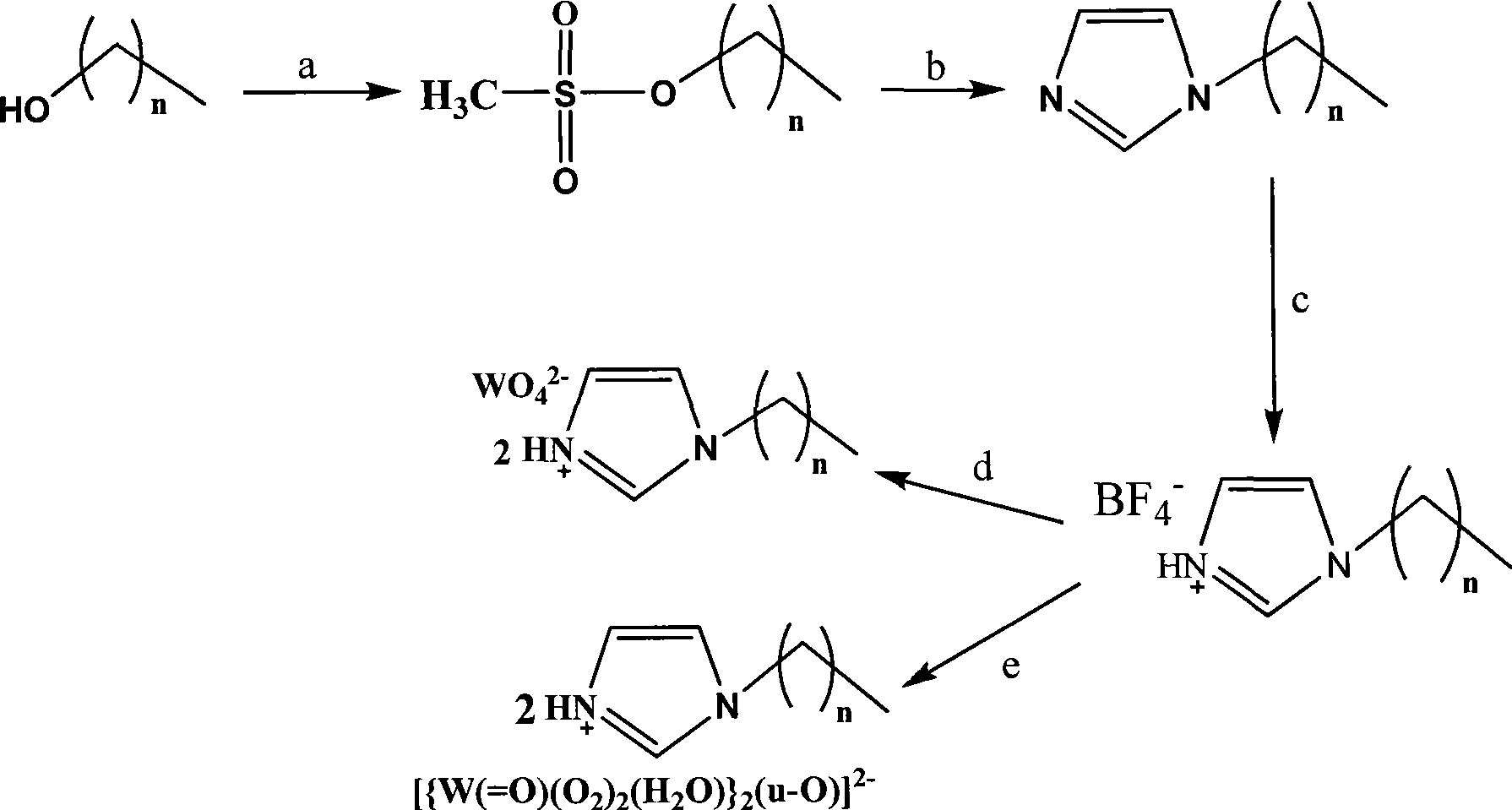
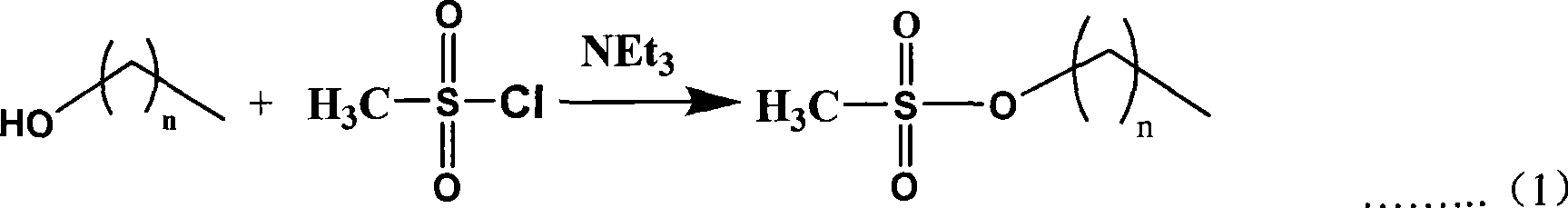Protonation alkyl imidazole tungstate ionic liquid and preparation method thereof
A technology of alkylimidazole tungstate and alkylimidazole tetrafluoroborate, which is applied in the field of preparation of protonated alkylimidazole tungstate ionic liquid, can solve research lag, few characteristic studies, melting point and viscosity increase and other issues, to achieve the effect of excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1: Protonated hexylimidazole tungstate ionic liquid [C 6 Im] 2 [WO 4 ] preparation
[0038] 1) Synthesis of hexyl methanesulfonate: all operations were carried out under nitrogen atmosphere. Under the condition of strong stirring in an ice bath, dissolve 3.7 ml of methanesulfonyl chloride (46 mmol) in anhydrous CH 2 Cl 2The solution was added dropwise to 30ml CH containing 5.0ml hexanol (40mmol) and 8ml triethylamine (56mmol) 2 Cl 2 middle. Stir at room temperature for 2 hours, stop the reaction and add ice cubes to cool, then add 20ml of ice water to the reaction solution to dilute. use CH 2 Cl 2 After extraction, the organic layer was washed with saturated ammonium chloride solution and distilled water respectively, and then the water in the organic phase was removed with sodium carbonate, and the solvent was removed by vacuuming under reduced pressure to obtain a nearly colorless oily hexyl methanesulfonate.
[0039] 2) Synthesis of hexylimidazole: ...
example 2
[0042] Example 2: Protonated octyl imidazolium tungstate ionic liquid [C 8 Im] 2 [WO 4 ] preparation
[0043] 1) Synthesis of octyl mesylate: all operations were carried out under nitrogen atmosphere. Under the condition of strong stirring in an ice bath, dissolve 3.7 ml of methanesulfonyl chloride (46 mmol) in anhydrous CH 2 Cl 2 The solution was added dropwise to 30 ml CH containing 6.3 ml octanol (40 mmol) and 8 ml triethylamine (56 mmol) 2 Cl 2 middle. Stir at room temperature for 2 hours, stop the reaction and add ice cubes to cool, then add 20ml of ice water to the reaction solution to dilute. use CH 2 Cl 2 After extraction, the organic layer was washed with saturated ammonium chloride solution and distilled water respectively, and then the water in the organic phase was removed with sodium carbonate, and the solvent was removed by vacuuming under reduced pressure to obtain a nearly colorless oily hexyl methanesulfonate.
[0044] 2) Synthesis of octylimidazole:...
example 3
[0047] Example 3: Protonated dodecyl imidazolium tungstate ionic liquid [C 12 Im] 2 [WO 4 ] preparation
[0048] 1) Synthesis of dodecyl methanesulfonate: all operations were carried out under nitrogen atmosphere. Under the condition of strong stirring in an ice bath, dissolve 3.7 ml of methanesulfonyl chloride (46 mmol) in anhydrous CH 2 Cl 2 The solution was added dropwise to 30ml CH containing 7.45g dodecanol (40mmol) and 8ml triethylamine (56mmol) 2 Cl 2 middle. Stir at room temperature for 2 hours, stop the reaction and add ice cubes to cool, then add 20ml of ice water to the reaction solution to dilute. use CH 2 Cl 2 After extraction, the organic layer was washed with saturated ammonium chloride solution and distilled water, and then the water in the organic phase was removed with sodium carbonate, and the solvent was removed by vacuuming under reduced pressure to obtain a colorless oily dodecyl methanesulfonate.
[0049] 2) Synthesis of dodecyl imidazole: 2.7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com