Compounds, formulations, and methods for treating or preventing inflammatory skin disorders
A skin disease, inflammatory technology, applied in the field of compounds for the treatment or prevention of inflammatory skin diseases, can solve problems such as inability to treat diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
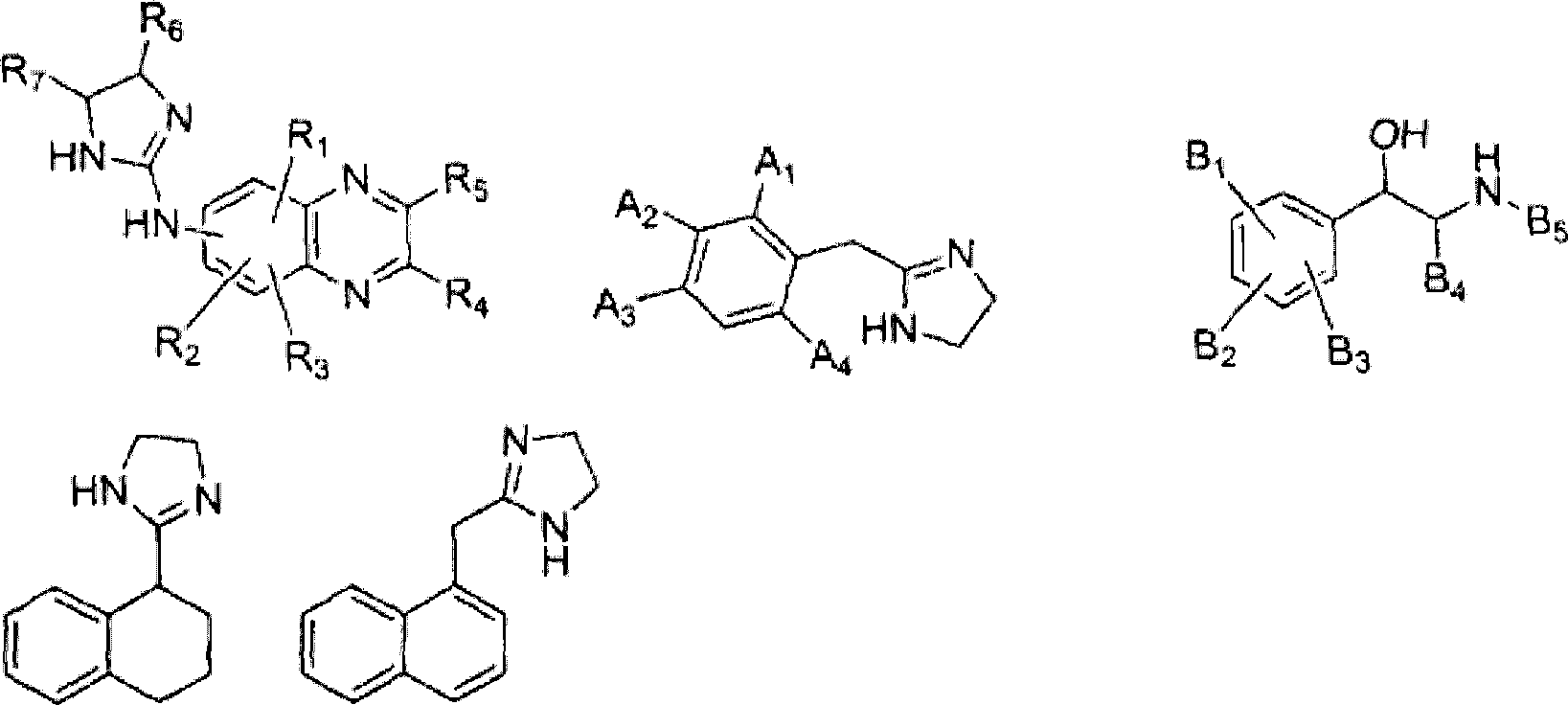
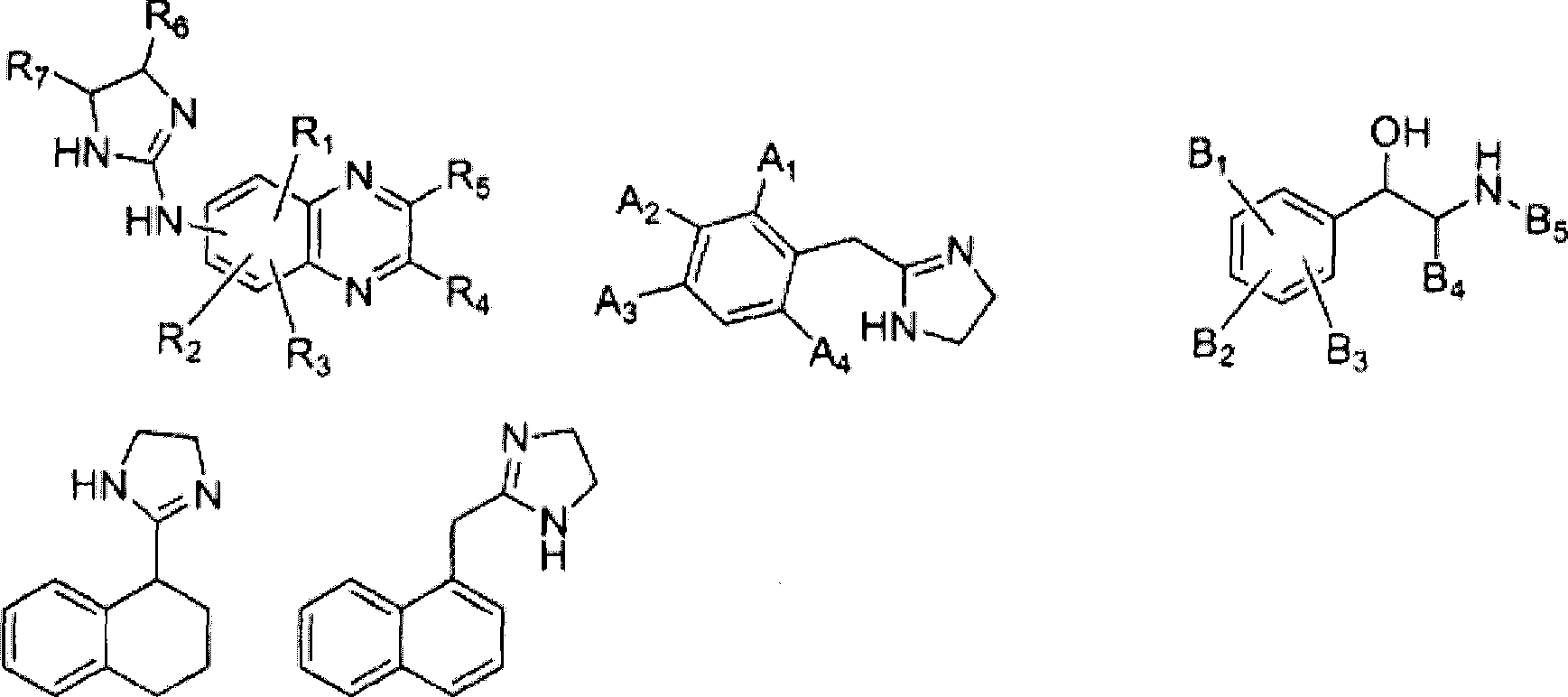
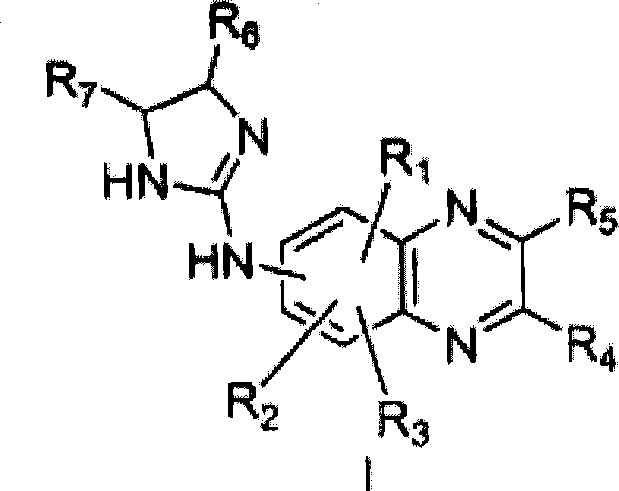
[0110] 1.7.1 Example 1: (5-bromo-quinoxalin-6-yl)-(4,5-dihydro-1H-imidazol-2-yl)-amine Synthesis
[0111] To a stirred solution of 6-amino-5-bromoquinoxaline hydrogen bromide (10 g) in distilled water (150 mL) was added thiophosgene (3 mL). The solution was stirred at room temperature for 2 h, and the resulting precipitate was collected by filtration, rinsed with water, and dried to give 5-bromo-6-isothiocyanato-quinoxaline.
[0112] 5-Bromo-6-isothiocyanato-quinoxaline (3.5 g) was directly dissolved in benzene (400 mL), and added dropwise to well-stirred ethylenediamine (15 g ) in the solution. In about two hours, an oil layer separated as the lower layer. The upper benzene layer was discarded, and the oil layer was washed with diethyl ether, and then dissolved in methanol (500 mL). The methanol solution was refluxed until evolution of hydrogen sulfide ceased. The methanol solution was concentrated under vacuum to a volume of about 100 mL, a yellow solid precipitated....
Embodiment 2
[0114] Aqueous aqueous topical formulations of the present invention include (5-bromo-quinoxalin-6-yl)-(4,5-dihydro-1H-imidazol-2-yl)-amine-L-tartrate (brimonide tartrate) (0.15wt.%); as a preservative (0.005%) (stabilized chlorine dioxide); and inert components: boric acid, calcium chloride, magnesium chloride, potassium chloride, pure water, sodium borate, sodium carboxymethylcellulose, sodium chloride, and with hydrochloric acid and / or sodium hydroxide to adjust the pH value to 5.6-6.6. The osmolality is 250-350mOsmol / kg.
Embodiment 3
[0116] Aqueous aqueous topical formulations of the present invention include (5-bromo-quinoxalin-6-yl)-(4,5-dihydro-1H-imidazol-2-yl)-amine-L-tartrate (brimonide tartrate) (0.15wt%); benzylamine chloride as a preservative; and inert components: boric acid, calcium chloride, magnesium chloride, potassium chloride, purified water, sodium borate, sodium carboxymethylcellulose, Sodium chloride, and adjust the pH value to 5.6-6.6 with hydrochloric acid and / or sodium hydroxide. The osmolality is 250-350mOsmol / kg.
[0117] 1.7.4 Example 4
[0118] Possible cream topical formulations of the invention are described in the table below.
[0119] Possible Cream Topical Formulations of the Invention (Hydrophilic Ointment USP)
[0120] components weight percentage Brimonidine tartrate 0.15% stearic acid 7% stearyl alcohol 5% cetyl alcohol 2% glycerin 10% sodium lauryl sulfate 1% Propylparaben 0.05% Methylparaben 0.25% D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com