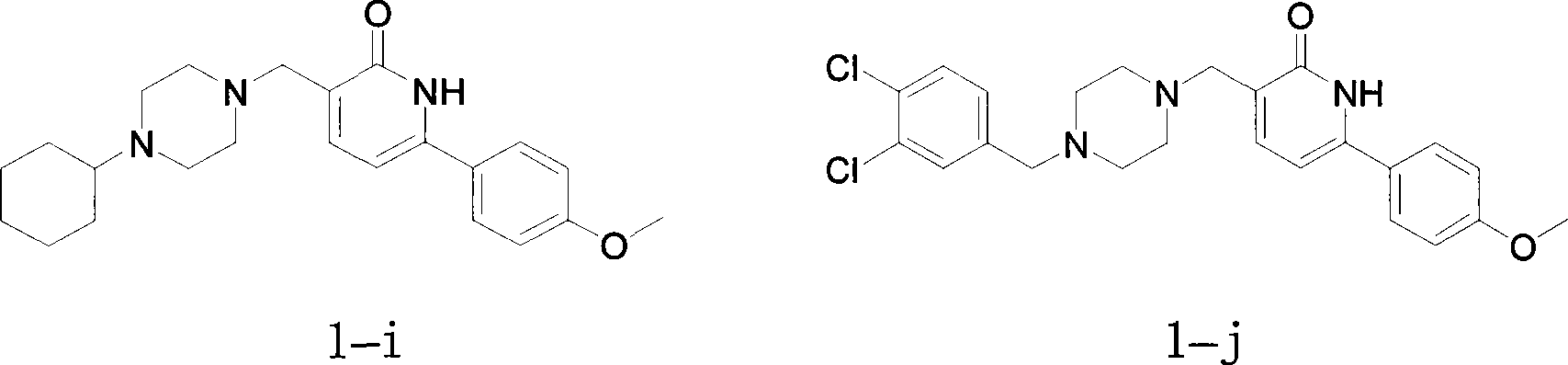
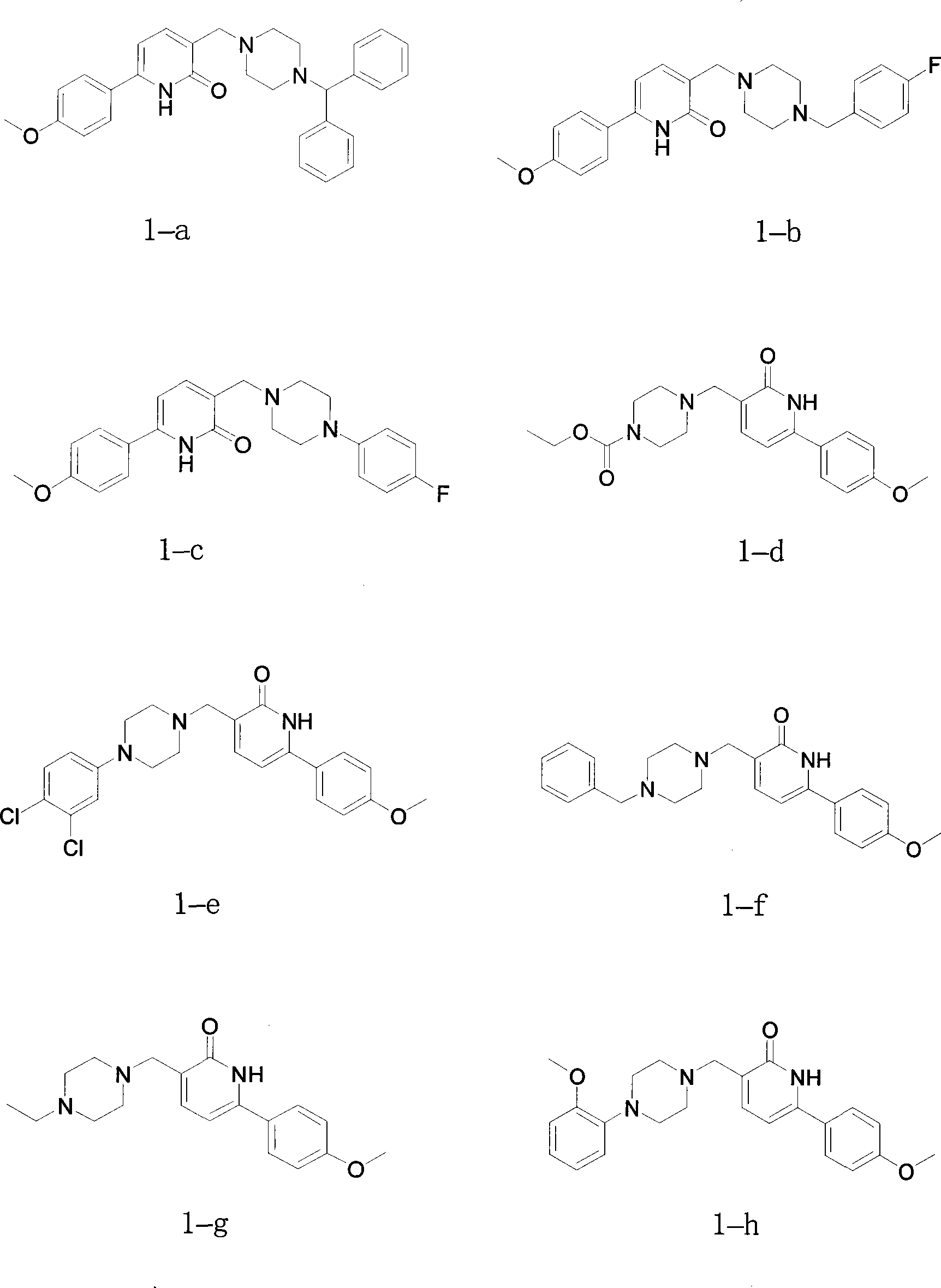Arylpyridone derivatives with acetylcholine esterase inhibition activity
A pyridone and pyridine technology, which is applied in the field of 6-aryl-3-substituted methylene-pyridone derivatives and their preparation, can solve the weakening of conduction signals, the reduction of acetylcholine concentration, and the disorder of acetylcholine synthesis and secretion, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0023] Preparation example 1: Preparation of starting material A (p-methoxyacetophenone):
[0024] p-Methoxyacetophenone
[0025] Methoxybenzene (10.8 g, 0.1 mol) was dissolved in 150 ml of dichloromethane, then anhydrous zinc chloride powder (26.8 g, 0.20 mol) was added, and acetic anhydride (15.3 g, 0.15 moles); after the dropwise addition, the reaction was slowly raised to room temperature for 7 hours, then the reactant was carefully poured into 600 ml of ice water, and extracted 3 times with ethyl acetate; the organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated The crude product was a colorless oil, and after short silica gel column chromatography, the starting material A (p-methoxyacetophenone): 13.1 g, yield 87%); white solid, melting point: 35-38°C. H NMR spectrum 1 H-NMR (400MHz, deuterated chloroform, δ ppm) δ 2.56 (single peak, 3H, COCH 3 ), 3.87 (single peak, 3H, OCH 3 ), 6.93 (doublet, 2H, J=8.4Hz, H-3,5), 7.94 (doublet, 2H, J...
preparation example 2
[0026] Preparation example 2: Preparation of intermediate B (3-cyano-6-(4-methoxyphenyl)-2H-pyridin-2-one):
[0027] 3-cyano-6-(4-methoxyphenyl)-2H-pyridin-2-one
[0028] Sodium metal (2.76 g, 120 mmol) was added to 250 ml of ether, 1 ml of ethanol was added dropwise, and starter A (p-methoxyacetophenone) (100 mmol) and ethyl formate were added dropwise under ice-cooling Ester (150 mmol) mixture, after the dropwise addition, the mixture was stirred for 15 minutes, then warmed up to room temperature and reacted for 1 hour. After diethyl ether was distilled off under reduced pressure, cyanoacetamide (12.6 g, 150 mmol) and water were added to the solid mixture (400ml). After the mixture was refluxed for 8 hours, cooled, acidified with acetic acid, and filtered to obtain a yellow solid, after drying, the initial product was recrystallized from ethanol to obtain intermediate B (3-cyano-6-(4-methoxyphenyl)- 2H-pyridin-2-one): Yield: 56%, pale yellow solid, melting point>250°C;...
preparation example 3
[0029] Preparation example 3: Preparation of intermediate C (3-cyano-6-(4-methoxyphenyl)-2-methoxypyridine):
[0030] 3-cyano-6-(4-methoxyphenyl)-2-methoxypyridine
[0031] Intermediate B (3-cyano-6-(4-methoxyphenyl)-2H-pyridin-2-one) (10 mmol) was prepared in N,N-dimethyldimethylacetal (DMFDMA) ( 1.8 g, 15 mmol) in N,N-dimethylformamide (50 mL) was heated to reflux overnight, and the mixture was poured into ice water. Produce a yellow solid precipitate, filter, wash the filter cake with a small amount of water, dry to obtain a crude product, recrystallize in ethanol to obtain compound intermediate C (3-cyano-6-(4-methoxyphenyl)-2 -methoxypyridine): yield: 89%; white solid; melting point: 137~138°C; R f (Petroleum ether / ethyl acetate 3:1) 0.46; H NMR 1 H-NMR (400MHz, deuterated chloroform, δppm): 3.89 (singlet, 3H, MeO-4'), 4.15 (singlet, 3H, MeO-2), 7.02 (doublet, 2H, J=8.4Hz, H-3', 5'), 7.36 (doublet, 1H, J=8.0Hz, H-5), 7.87 (doublet, 1H, J=8.0Hz, H-4), 8.04 (doublet, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com