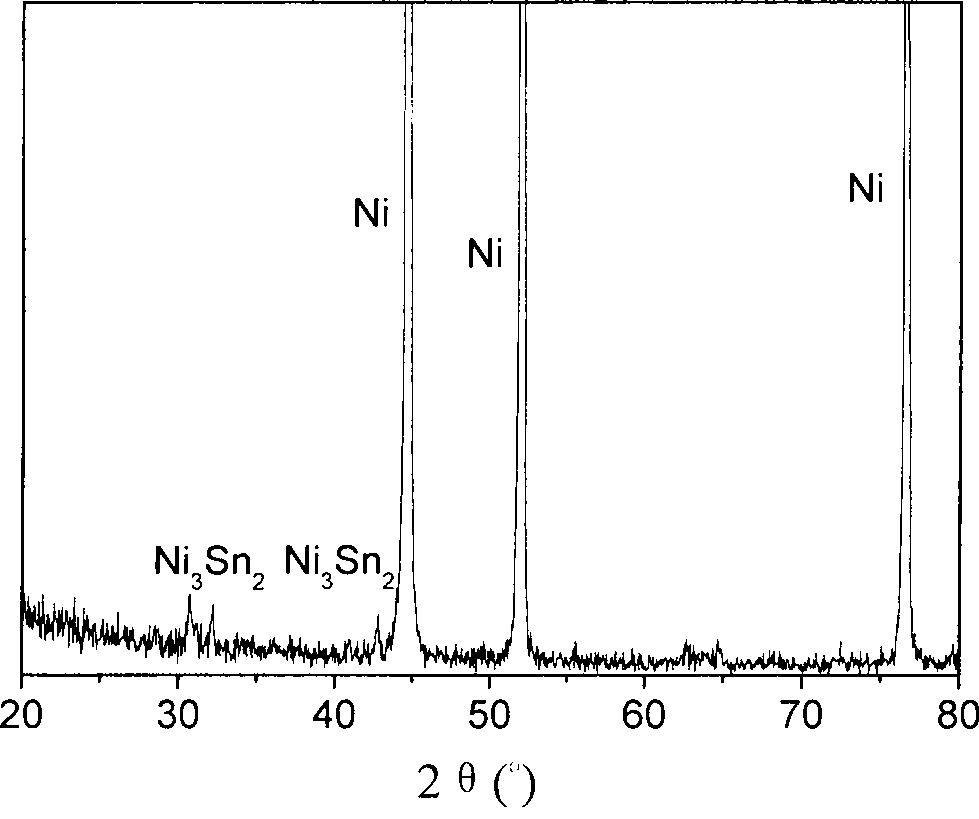
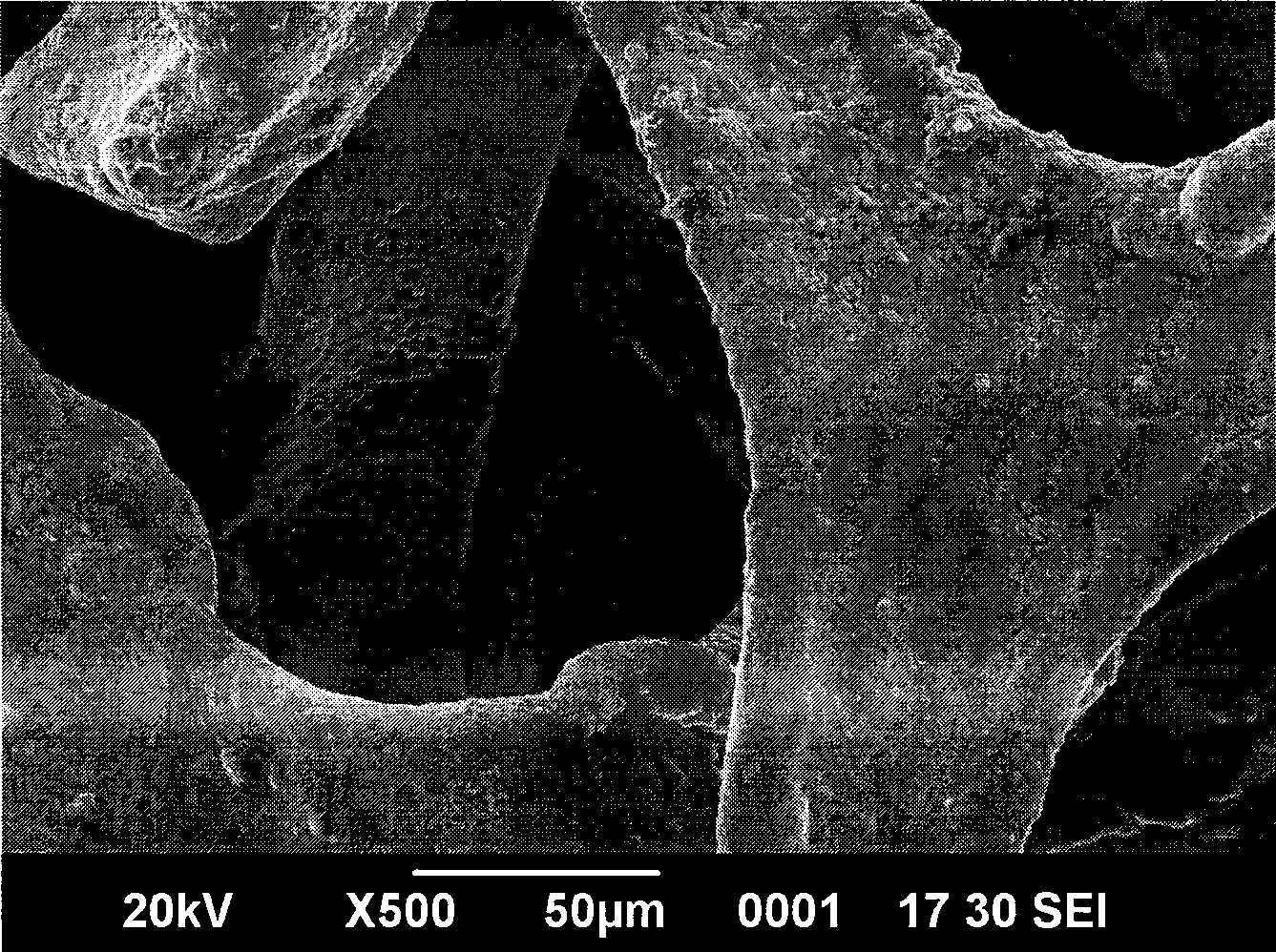
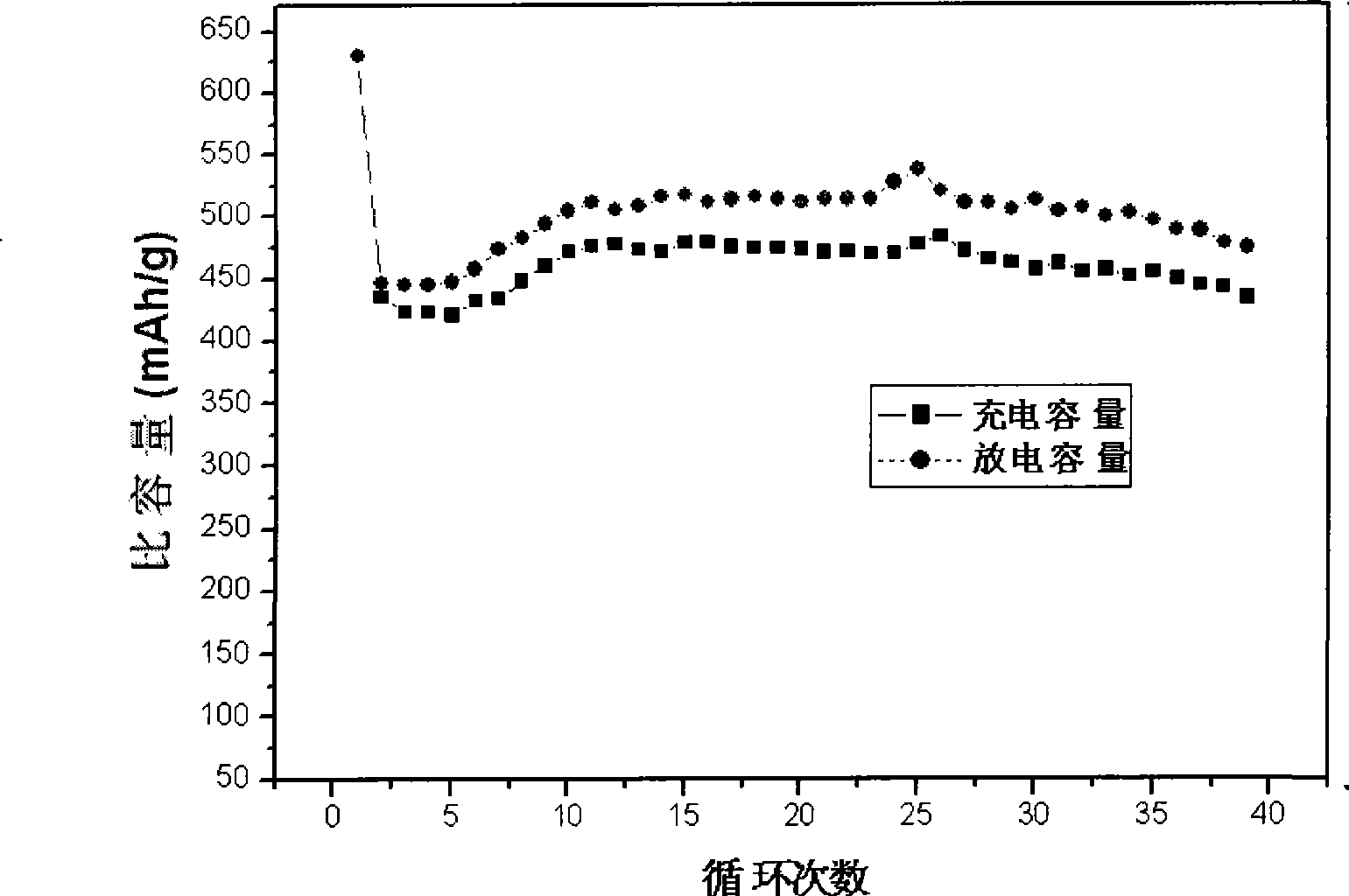
Method for preparing nickeltin thin film electrode for lithium ion battery through electrochemical deposition
A lithium-ion battery, nickel-tin alloy technology, applied in battery electrodes, electrode manufacturing, circuits, etc., can solve problems such as large volume expansion and complex polystyrene ball preparation process, and achieve high capacity, good cycle performance, and easy scale. The effect of chemical production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of electrolyte solution of nickel-tin alloy
[0032] a. In a 100ml beaker, dissolve 15g of SnCl with 20ml of pure hydrochloric acid 2 .2H 2 O is heated and dissolved, and diluted to 100ml after dissolution;
[0033] b. In a 100ml beaker, heat and dissolve 15g NiCl with 100ml distilled water 2 .6H 2 O;
[0034] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 .
[0035] d. SnCl first 2 solution added to K 4 P 2 o 7 solution, then NiCl 2 solution added to K 4 P 2 o 7 Then add 5g of L-glutamic acid to the solution, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a green nickel-tin electrolyte.
[0036] 2. The electrochemical deposition system is composed of nickel foam as the cathode, graphite electrode as the anode, DC power supply and electrolytic cell containing nickel-tin metal ions;
[0037] 3. Electrochemical deposition is ...
Embodiment 2
[0043] 1. Preparation of electrolyte solution of nickel-tin alloy
[0044] a. In a 100ml beaker, dissolve 15g Na with 20ml pure hydrochloric acid 2 SnO 3 .3H 2 O is heated and dissolved, and diluted to 100ml after dissolution;
[0045] b. In a 100ml beaker, heat and dissolve 15g NiCl with 100ml distilled water 2 .6H 2 O;
[0046] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 .
[0047] d. Na 2 SnO 3 solution added to K 4 P 2 o 7 solution, then NiCl 2 solution added to K 4 P 2 o 7 Then add 5g of L-glutamic acid to the solution, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a green nickel-tin electrolyte.
[0048] 2. The electrochemical deposition system is composed of nickel foam as the cathode, graphite electrode as the anode, DC power supply and electrolytic cell containing nickel-tin metal ions;
[0049] 3. Electrochemical deposition is carr...
Embodiment 3
[0052] 1. Preparation of electrolyte solution of nickel-tin alloy
[0053] a. In a 100ml beaker, dissolve 15g of SnCl with 20ml of pure hydrochloric acid 2 .2H 2 O is heated and dissolved, and diluted to 100ml after dissolution;
[0054] b. In a 100ml beaker, heat and dissolve 15g NiCl with 100ml distilled water 2 .6H 2 O;
[0055] c. In a 500ml beaker, heat and dissolve 200g K with 200ml distilled water 4 P 2 o 7 ;
[0056] d. SnCl first 2 solution added to K 4 P 2 o 7 solution, then NiCl 2 solution added to K 4 P 2 o 7 Then add 5g of L-glutamic acid to the solution, adjust the pH of the solution to 7-9 with ammonia water, and finally set the volume to 500ml with a volumetric flask to obtain a green nickel-tin electrolyte.
[0057] 2. The electrochemical deposition system is composed of nickel foam as the cathode, graphite electrode as the anode, DC power supply and electrolytic cell containing nickel-tin metal ions;
[0058] 3. Electrochemical deposition is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com