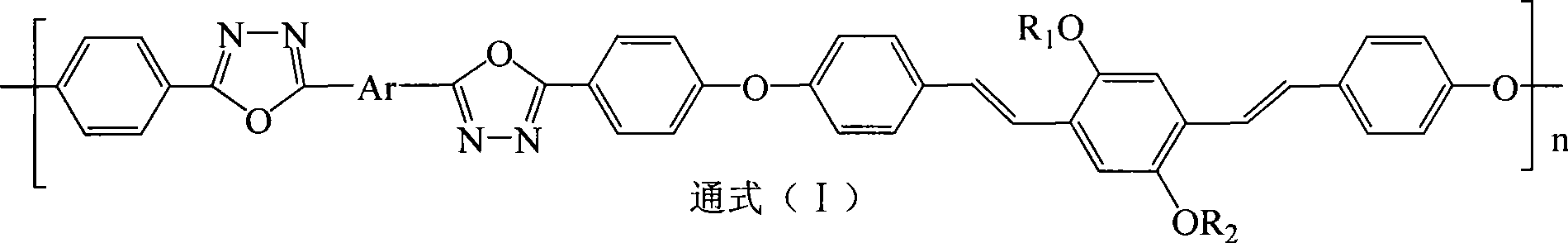
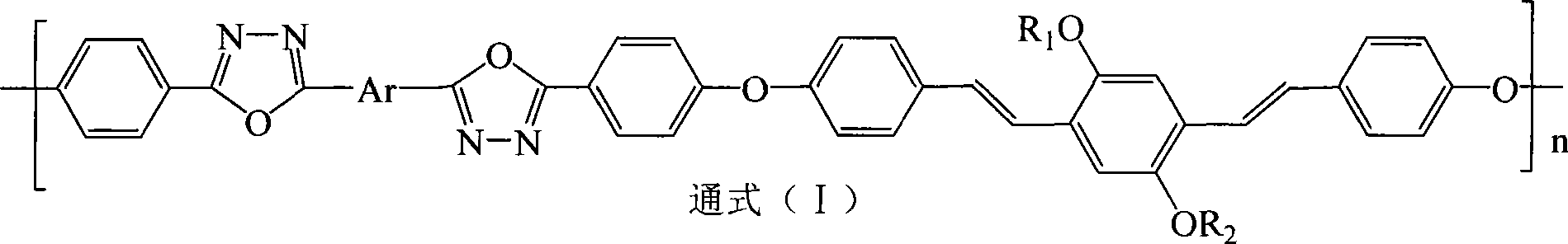
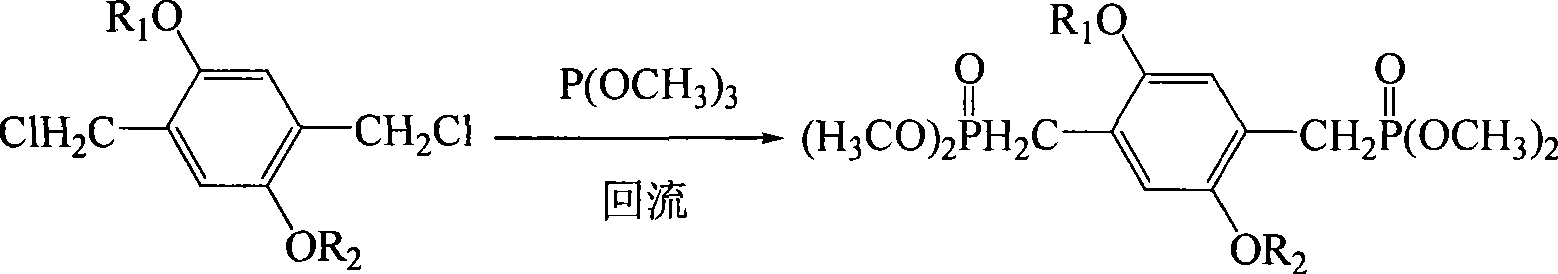
Electric charge transmission type conjugated polymer emitting blue light
A conjugated polymer and charge transport technology, applied in the field of polymers, can solve the problems of high preparation method and technical difficulty, poor controllability of synthesis preparation process, complex structure of synthetic raw materials, etc., and achieves classic preparation method, pure luminescence color. , the effect of high degree of commercialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 (I-1-1) conjugated polymer
[0030]
[0031] Step 1 Preparation of 2,5-dilauryloxy-1,4-bis[2-(4-hydroxyphenyl)vinyl]benzene In a four-necked reaction flask equipped with a stirrer, add 0.012 moles of p-hydroxybenzene Formaldehyde and 0.015 moles of potassium carbonate were stirred and dispersed evenly with 200 ml of 1,4-epoxyhexane, and the temperature was raised to 50° C. for 2 hours. Thereafter, 0.005 moles of 2,5-dilauryloxy-1,4-bis(dimethoxyphosphorylmethyl)benzene and 0.015 moles of sodium tert-butoxide were sequentially added, and the temperature was raised to 85° C. for 6 hours under reflux to complete the reaction. The reaction product system was cooled to room temperature, poured into 1000 ml of 10% by mass aqueous acetic acid solution, and the precipitate was collected. The filter cake was recrystallized with a mixed solvent of tetrahydrofuran / ethanol (V / V=4:1), and dried in vacuum to obtain light yellow 2,5-dilauryloxy-1,4-...
Embodiment 2
[0044] The preparation of embodiment 2 (1-2-1) conjugated polymer
[0045]
[0046] According to the preparation method and steps in Example 1 of the present invention, 2,5-bis[5-(4-iodophenyl)oxadiazolyl-2-]thiophene in Step 2 of Example 1 was replaced with 2,6 -bis[5-(4-iodophenyl)oxadiazolyl-2-]pyridine, the (I-2-1) conjugated polymer can be prepared, the appearance of the polymer is yellow powder, and DSC records vitrification The temperature Tg=77°C, and the temperature at which the weight loss in nitrogen was 5% was 405°C as measured by TGA.
[0047] Infrared spectrum (KBr pellet, cm -1 ): 2931, 2827, 1677, 1613, 1538, 1447, 1106, 904.
[0048] UV-Vis absorption spectrum (CHCl 3 solution): λ max = 412nm.
[0049] Fluorescence spectrum (CHCl 3 solution): λ max = 493nm.
[0050] (I-2-1) Solubility Table of Conjugated Polymers
[0051] Solvent name Glacial acetic acid Ethanol Ethylene glycol monomethyl ether Chloroform Toluene Dimethyl sulfoxide S...
Embodiment 3
[0052] The preparation of embodiment 3 (I-1-2) conjugated polymer
[0053]
[0054] According to the preparation method and steps in Example 1 of the present invention, the 2,5-dilauryloxy-1,4-bis[2-(4-hydroxyphenyl)vinyl]benzene in Step 1 of Example 1 was replaced with It is 2,5-diisooctyloxy-1,4-bis[2-(4-hydroxyphenyl)vinyl]benzene, and (I-1-2) conjugated polymer can be prepared. The appearance of the polymer is It is a golden yellow powder, the glass transition temperature Tg=73°C as measured by DSC, and the temperature at which the weight loss in nitrogen is 5% is 385°C as measured by TGA.
[0055] Infrared spectrum (KBr pellet, cm -1 ): 2928, 2832, 1685, 1611, 1438, 1126, 906.
[0056] UV-Vis absorption spectrum (CHCl 3 solution): λ max = 412nm.
[0057] Fluorescence spectrum (CHC1 3 solution): λ max = 492nm.
[0058] (I-1-2) Solubility Table of Conjugated Polymers
[0059] Solvent name Ethanol Ethylene glycol monomethyl ether Chloroform Toluene Dime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com