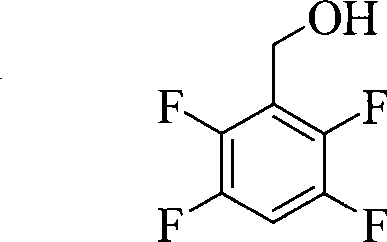
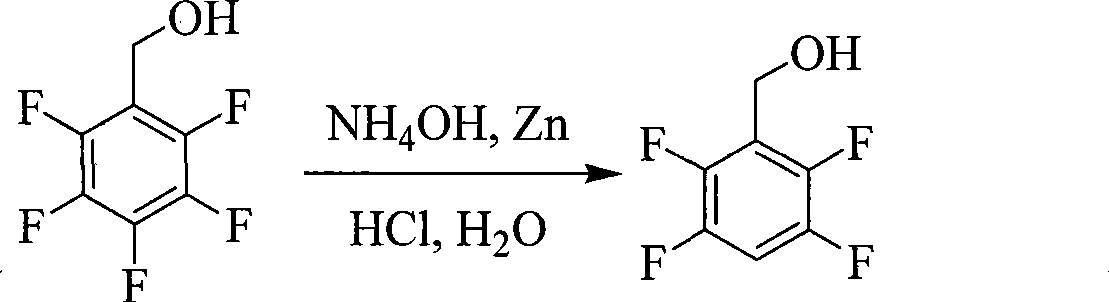
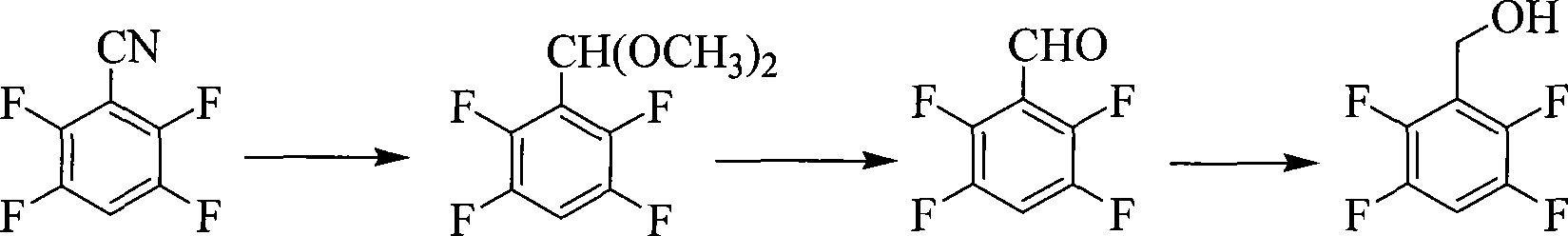
Preparation of 2,3,5,6-tetrafluorobenzyl alcohol
A technology of tetrafluorobenzyl alcohol and tetrafluorobenzonitrile, which is applied in the field of synthesis of chemical intermediates, can solve problems such as increasing costs and increasing environmental pollution, and achieves the effects of reducing by-products, reducing pollution, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-1
[0066] Weigh 33.3g of pentafluorobenzonitrile, 1.6g of RTH-311 type Raney-Ni (produced by Datong General Chemical Co., Ltd.), 83.0mL of methanol, and 17.3g of concentrated sulfuric acid into a 250mL autoclave, replace the gas with nitrogen three times, pass h 2 When the pressure is 25 atm, the temperature is raised to 55° C. After reacting for 14 hours, sampling analysis is performed, the raw materials disappear, the insoluble matter is filtered out, and the solvent is recovered under reduced pressure to obtain 48.8 g of sulfate salt of pentafluorobenzylamine, content: 98.5% (HPLC), Yield: 94.4%.
Embodiment I-2
[0068] Weigh 30.0g of pentafluorobenzonitrile, 1.5g of RTH-411 type Raney-Ni (produced by Datong General Chemical Co., Ltd.), 120mL of ethanol, and 26.5g of trifluoroacetic acid into a 250mL autoclave, replace the gas with nitrogen three times, and pass h 2 When the pressure is 20 atm, the temperature is raised to 60° C., reacted for 16 hours, sampling analysis, the raw material disappears, the insoluble matter is filtered out, and the solvent is recovered under reduced pressure to obtain 45.5 g of trifluoroacetate of pentafluorobenzylamine, content: 98.2% (HPLC ), yield: 94.0%.
Embodiment I-3
[0070] Weigh 20.5g of pentafluorobenzonitrile, 0.2g of 10% Pd / C (produced by Baoji Ruike Pharmaceutical Chemical Co., Ltd.), 70mL of THF, and 9.5g of acetic acid into a 250mL autoclave, replace the gas with nitrogen three times, and pass H 2 When the pressure is 10 atm, the temperature is raised to 55° C. After reacting for 10 hours, sampling analysis is carried out, the raw materials disappear, the insoluble matter is filtered out, and the solvent is recovered under reduced pressure to obtain 25.6 g of acetate of pentafluorobenzylamine, content: 97.9% (HPLC) , Yield: 93.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com