Polyarylether polymer containing amino-benzene lateral group and method for synthesizing the same
A synthesis method and technology of aminophenyl hydroquinone, applied in the field of polymers, can solve the problems of reducing amino activity, affecting the reaction, large steric hindrance, etc., and achieving low water swelling rate, good film-forming property, and good flexibility. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
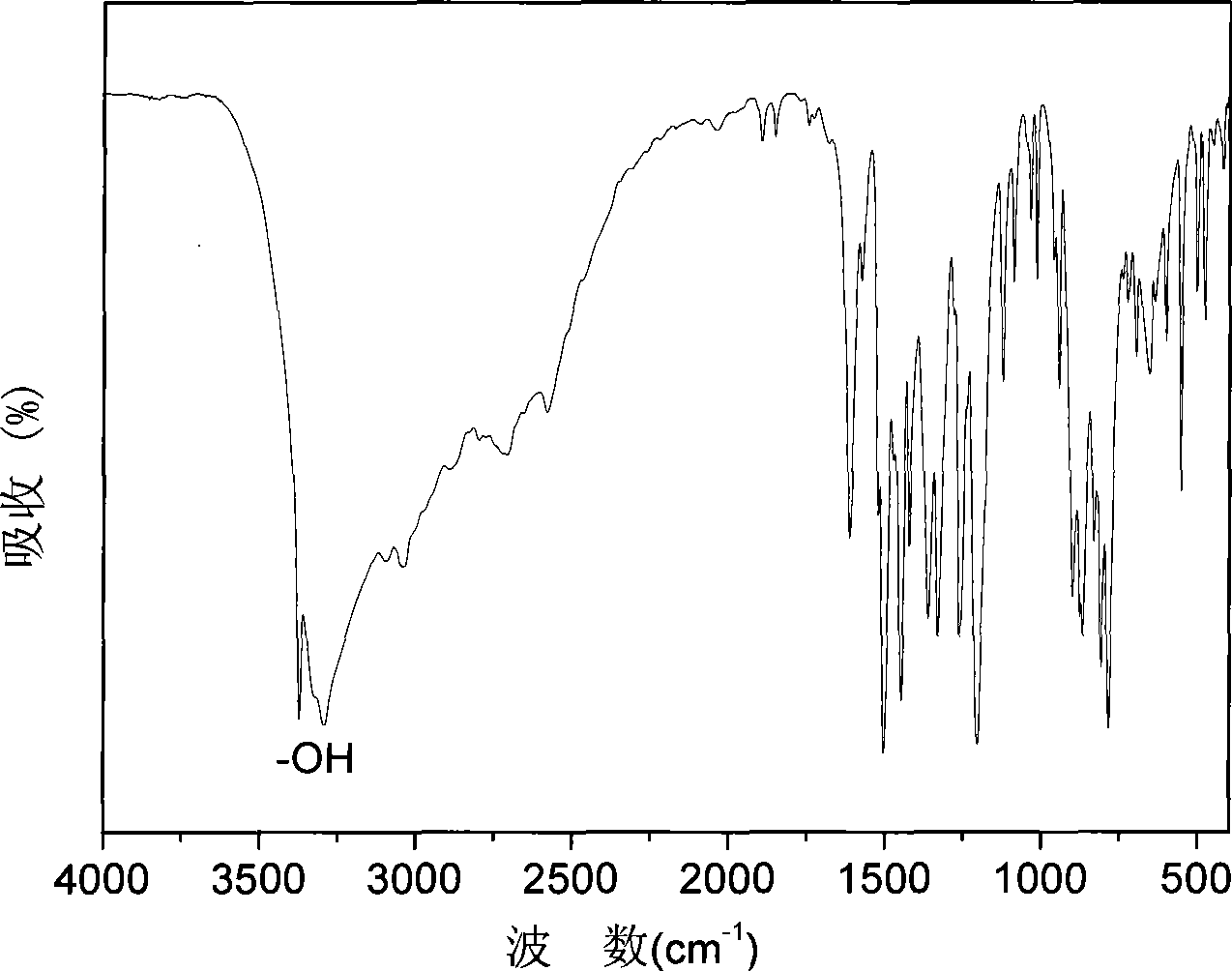
[0025] The raw material that the present invention adopts---the synthetic method that contains aminophenyl hydroquinone monomer, can divide three-step reaction and carry out:
[0026] First step response:
[0027] Using 4-nitroaniline as the raw material, add the raw material, ethanol, and water into the container D, heat and stir mechanically, cool down to room temperature after dissolving, add hydrochloric acid, react for 0.5-1 hour, and dilute the sub- Sodium nitrate aqueous solution is dropped into the reaction system, the temperature is controlled at 0-10 ° C, the dropping time and the reaction time are 2-6 hours in total, and the intermediate product A is obtained, wherein the raw materials, concentrated hydrochloric acid, sodium nitrite, ethanol, and water mole The ratio is 1:4-6:1:5-100:5-100.
[0028] Second step reaction:
[0029] Add benzoquinone, sodium bicarbonate and water into container E, stir vigorously, control the temperature at 3-15°C, pour or drop the in...
Embodiment 2
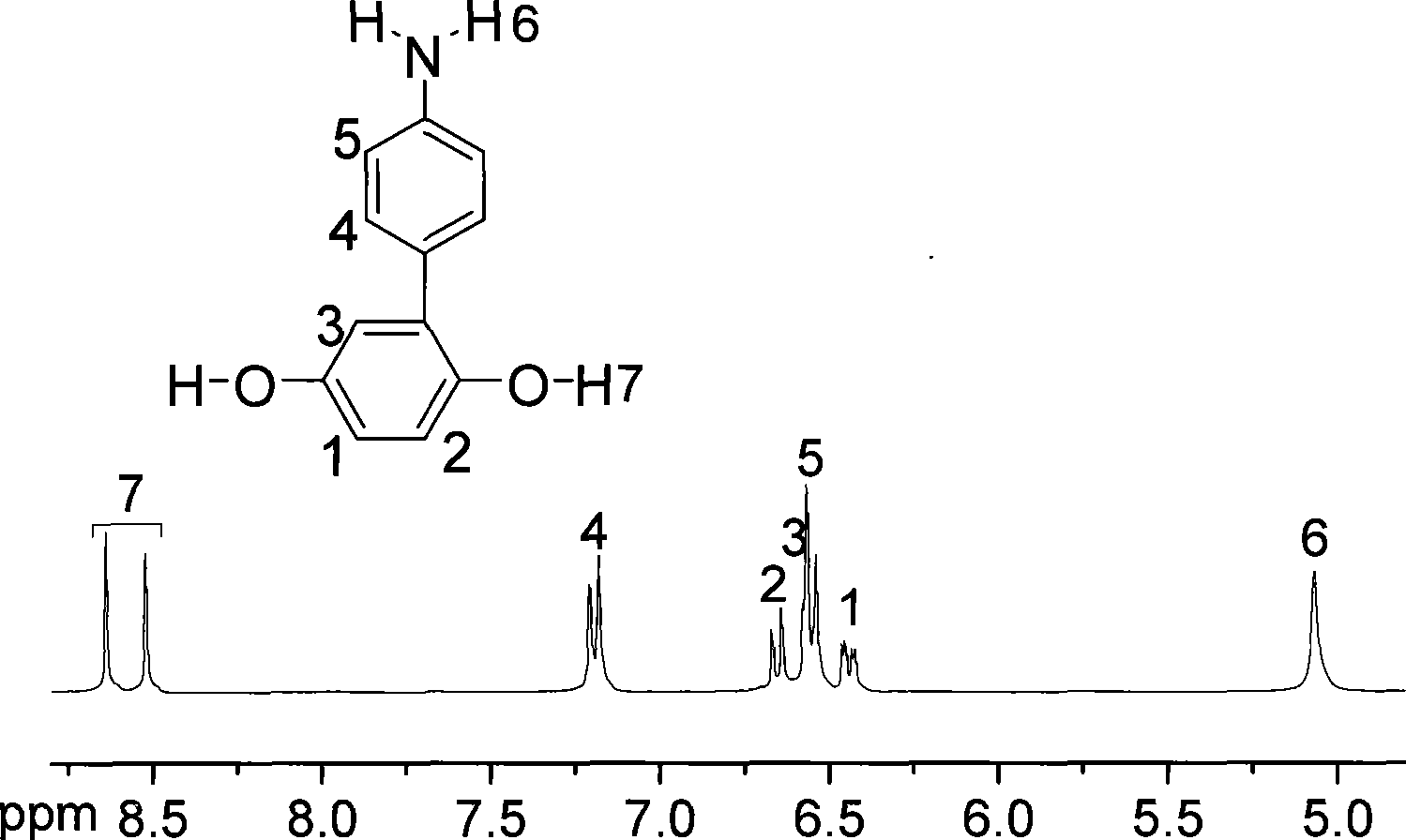
[0034] 2.0122 grams (0.01mol) of 4-aminophenylhydroquinone prepared by the method of Example 1, 2.1820 grams (0.01mol) of 4,4'-difluorobenzophenone and 1.6585 grams of anhydrous potassium carbonate, 22.5 Put 1ml sulfolane and 20ml toluene into a 100ml three-necked flask equipped with a water device, blow nitrogen, heat up to toluene reflux and stir, reflux for 1.5 to 2 hours, remove toluene, raise the temperature to 180°C, and continue the reaction at about 180°C for 4~ After 5 hours, the polymerization solution was precipitated in water, pulverized, washed, and dried to obtain a brown powder of polyetheretherketone with a 4-aminophenyl structure. The glass transition temperature measured by DSC was 203°C. The molecular formula is as follows:
[0035]
[0036] Where n=100-220.
Embodiment 3
[0038] The method is the same as in Example 2, and the amount of the monomer is enlarged tenfold for reaction: 20.122 grams (0.1mol) of 4-aminophenylhydroquinone, 21.820 grams (0.1mol) of 4,4'-difluorobenzophenone and 16. Put 5852 grams of anhydrous potassium carbonate, 230ml sulfolane, and 60ml toluene into a 500ml three-necked flask equipped with a water dispenser, ventilate nitrogen, heat up to toluene reflux and stir, reflux for 3 to 4 hours, remove toluene, and heat up to 190°C. Continue to react at about 190°C for 7-9 hours, precipitate the polymerization solution in water, pulverize, wash, and dry to obtain a brown powder of polyetheretherketone with the same 4-aminophenyl structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com