Preparation method of alkali metal tantalate composite visible-light photocatalyst for hydrogen production from photodissociation of water
A photocatalyst and alkali metal technology, which is applied in the field of preparation of alkali metal tantalate-based composite photocatalysts for the production of visible light from water splitting, can solve problems such as nitrogen doping of alkali metal tantalate, and achieve good visible absorption, high Visible light catalytic activity, the effect of good visible light catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 0.2g of P123 surfactant in 20mL of 7molL -1 of hydrochloric acid solution. After stirring for 1h, take 1g of tantalum pentachloride and add it, and then stir for 1h. Ammonia solution with a mass concentration of 1.25% was added dropwise while stirring until the pH of the solution was pH=7. Stop stirring, and after precipitation, take all the precipitate and 60 mL of the solution, add 5 g of sodium hydroxide, and stir for 30 min to completely dissolve it. All of the above procedures were carried out in an ice bath. It was transferred to an 80mL reactor and subjected to hydrothermal treatment at 140°C for 10h. After cooling, the product was obtained, and the product was washed with deionized water several times to remove sodium hydroxide, and then dried at 60° C. for 24 hours. Then it is heat-treated for 1h under the condition of passing oxygen at 400°C (the heating rate of the furnace is 5Kmin -1 ), remove a small amount of P123 remaining in the product, an...
Embodiment 2
[0029] Dissolve 0.2g of CTAB surfactant in 20mL of 6molL -1 of hydrochloric acid solution. After stirring for 2h, take 1g of tantalum pentachloride and add it, and then stir for 1h. Ammonia solution with a mass concentration of 1.25% was added dropwise while stirring until the pH of the solution was pH=8. Stop stirring, and after precipitation, take all the precipitate and 60 mL of the solution, add 10 g of potassium hydroxide, and stir for 30 min to completely dissolve it. All of the above procedures were carried out in an ice bath. It was transferred to an 80mL reactor and subjected to hydrothermal treatment at 120°C for 15h. After cooling, the product was obtained, and the product was washed with deionized water several times to remove potassium hydroxide, and then dried at 60° C. for 24 hours. Then it is heat-treated for 1h under the condition of passing oxygen at 400°C (the heating rate of the furnace is 5Kmin -1 ), remove a small amount of CTAB remaining in the prod...
Embodiment 3
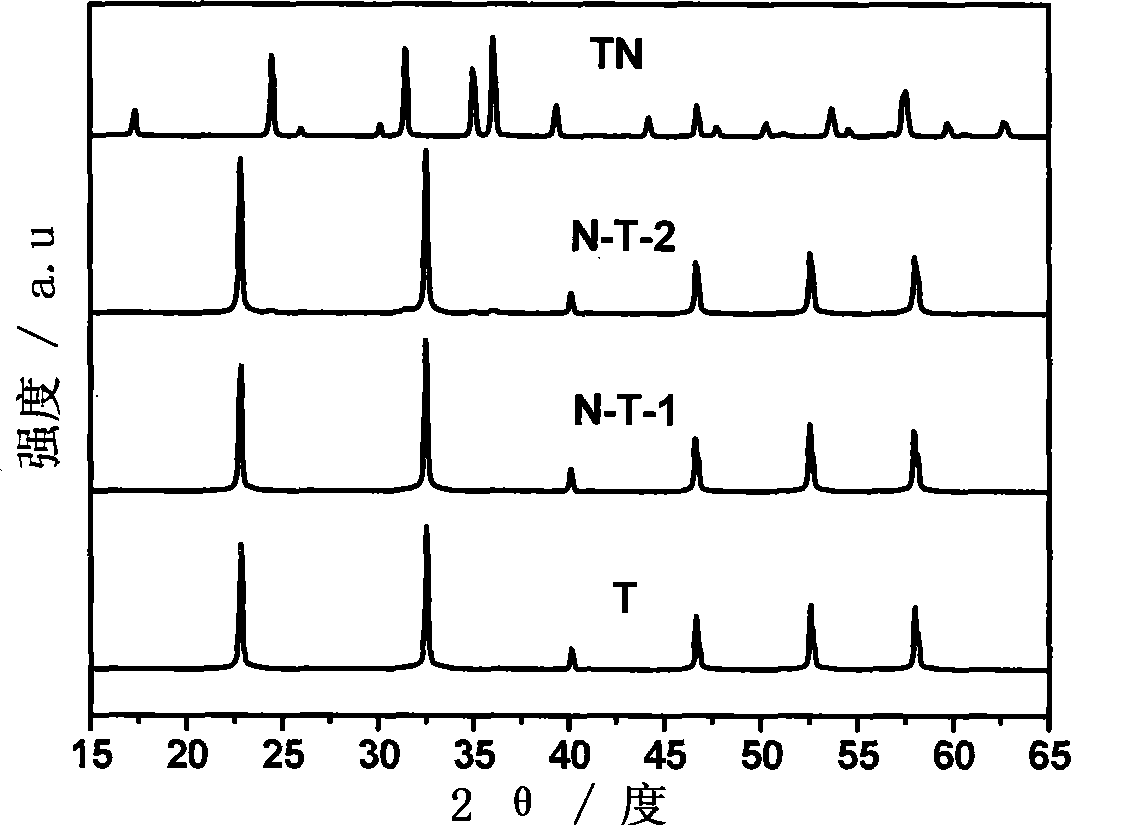
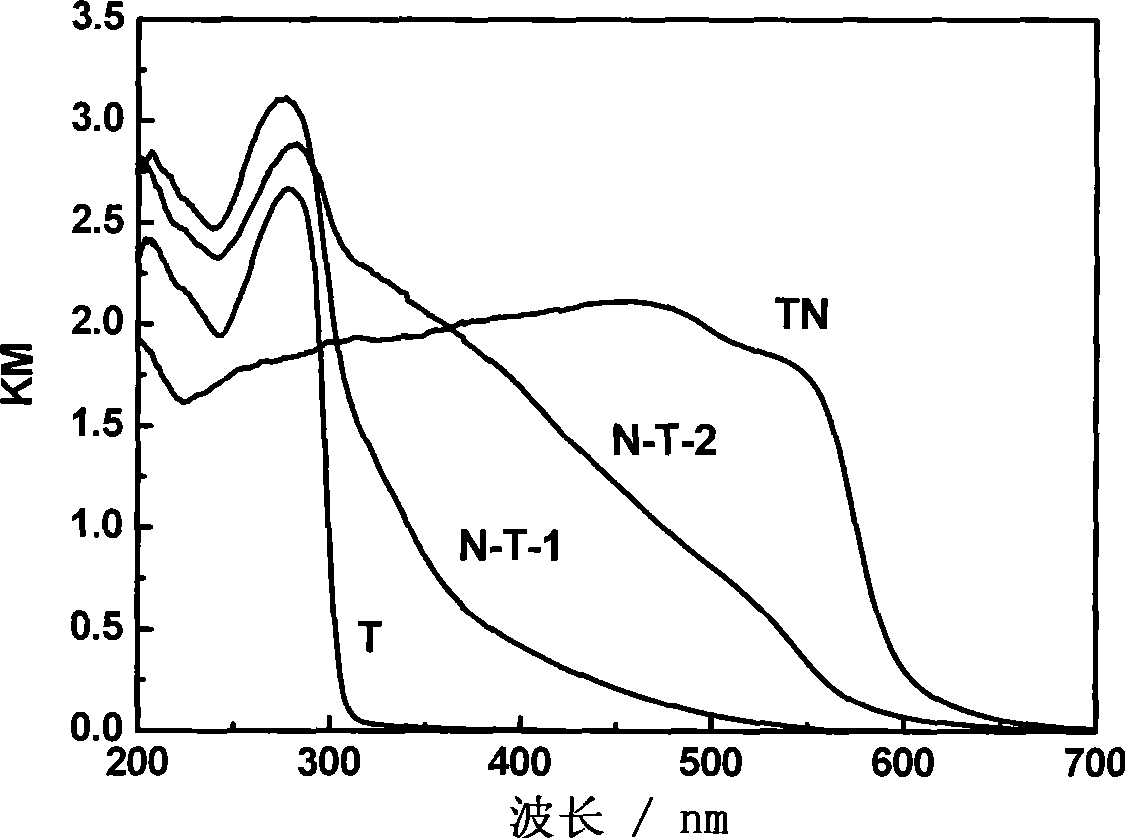
[0031]Add 0.2g of P123 into 60mL of deionized water (pH=6), stir for 1h and then add 0.8g of Ta 2 o 5 , and stirred for 1h. Then add 2g NaOH and stir for 30min. All the above steps are carried out in ice bath. It was transferred to an 80mL reactor and subjected to hydrothermal treatment at 140°C for 20h. After cooling, the product was obtained, and the product was washed with deionized water several times to remove sodium hydroxide. Then dry at 60°C for 24h, then heat-treat it at 400°C for 1h under the condition of oxygen flow (the heating rate of the furnace is 5Kmin -1 ), remove a small amount of P123 remaining in the product, and finally obtain pure sodium tantalate. Get the sodium tantalate of the hydrothermal synthesis of 0.5g, under 850 ℃ (heating rate 10Kmin -1 ), through ammonia gas 25mLmin -1 The flow rate, nitriding treatment 10h, obtain nitrogen-doped sodium tantalate, the nitrogen doping amount of this embodiment is 4wt%, its characterization and performance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com