Preparation of quinoxaline derivatives
A technology of quinoxaline derivatives, which is applied in the field of preparation of quinoxaline derivatives, can solve the problems of long reaction time, harsh reaction conditions, use of special instruments or devices, etc. The effect of mild conditions and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
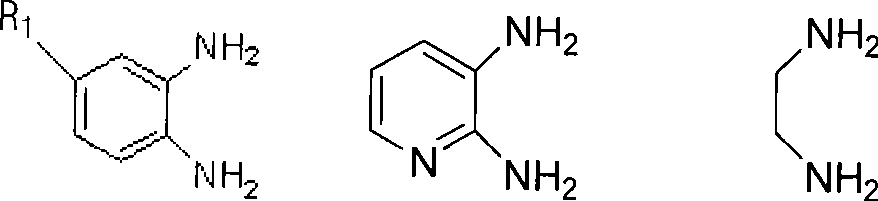
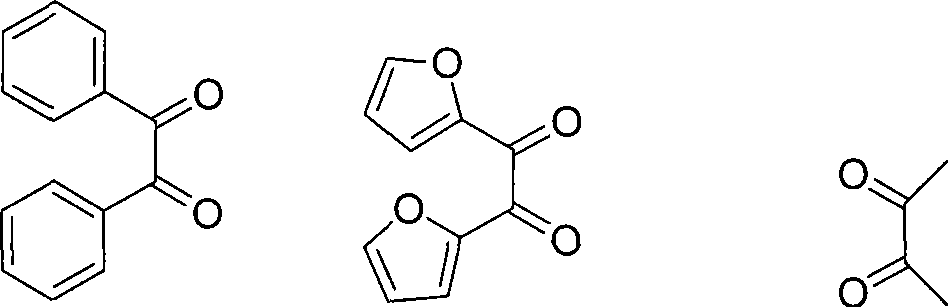
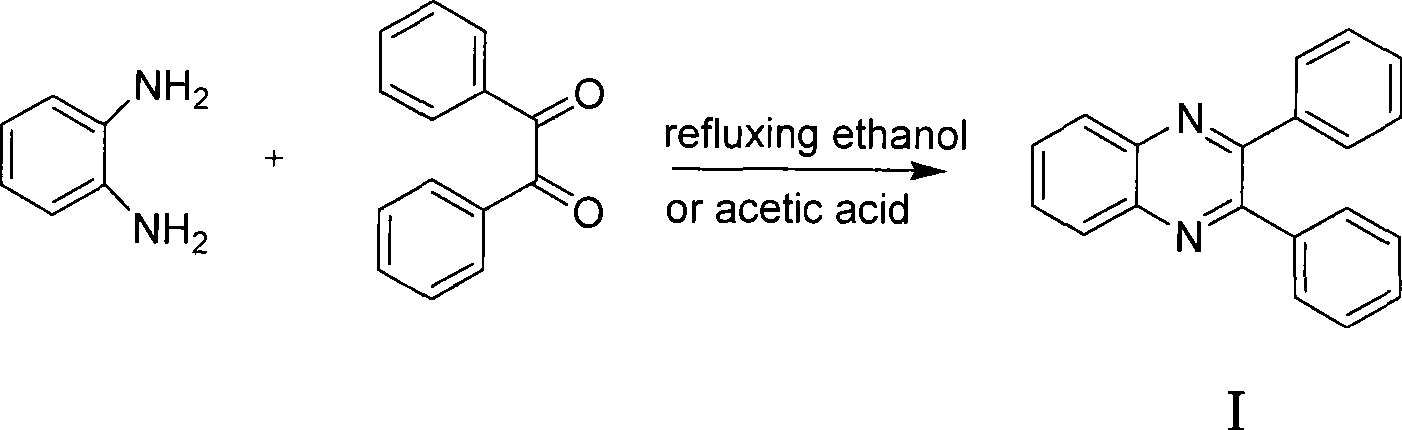
[0034] Add o-phenylenediamine (108mg, 1mmol), diphenyl ketone (210mg, 1mmol), Amberlyst-15 (52mg, 0.24mmol) respectively in a 100mL three-neck round bottom flask equipped with a magnet, reflux condenser and thermometer , 24mL of water, started stirring and controlled the temperature at 25°C for 19 minutes, during which the reaction progress was monitored by TLC, and ethyl acetate-petroleum ether (v / v=1:5) was used as a developing solvent. After the reaction is finished, add 20 mL of ethyl acetate, stir well and then filter, so that the catalyst can be recovered. The organic layer in the above filtrate was collected, dried with anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain the crude product of 2,3-diphenylquinoxaline. The crude product can be further purified by using ethyl acetate-petroleum ether (v / v=1:6) as an eluent through a flash chromatography column to obtain pure 2,3-diphenylquinoxaline.
[0035] 2,3-Diphenyl-quinoxaline (the product in No. 1)...
Embodiment 2
[0037] Add 4-methyl o-phenylenediamine (134mg, 1.1mmol), diphenyl ketone (210mg, 1mmol) and NKC-9 dry Hydrogen-catalyzed resin Styrene-DVB (76mg, 0.35mmol), 24mL water, started stirring and controlled the temperature at 50°C, reacted for 17 minutes, during which the reaction progress was monitored by TLC, and ethyl acetate-petroleum ether (v / v=1: 5) As a developing agent. After the reaction is finished, add 20 mL of ethyl acetate, stir well and then filter, so that the catalyst can be recovered. The organic layer in the above filtrate was collected, dried with anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain the crude product of 6-methyl-2,3-diphenylquinoxaline. The crude product was recrystallized from absolute ethanol to obtain pure 6-methyl-2,3-diphenylquinoxaline.
[0038]6-methyl-2,3-diphenylquinoxaline (the product in No. 2): m.p.116-117°C; 1 HNMR (CDCl 3 , 300MHz) δ (ppm): 8.1 (d, 1H), 7.96 (s, 1H), 7.63 (dd, 1H), 7.5 (m, 4H), 7.35 (m, 6H), 2.6...
Embodiment 3
[0040] Add 4-nitro-o-phenylenediamine (183mg, 1.2mmol), benzophenone (210mg, 1mmol), Amberlyst-15 ( 102mg, 0.47mmol) (for secondary application), 24mL water, start stirring and control the temperature at 65°C, react for 54 minutes, monitor the reaction progress with TLC during the reaction, use ethyl acetate-petroleum ether (v / v=1:5) as a developing agent. After the reaction is finished, add 20 mL of ethyl acetate, stir well and then filter, so that the catalyst can be recovered. The organic layer in the above filtrate was collected, dried with anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain the crude product of 6-nitro-2,3-diphenylquinoxaline. The crude product was further purified by flash chromatography using ethyl acetate-petroleum ether (v / v=1:6) as eluent to obtain pure 6-nitro-2,3-diphenylquinoxaline.
[0041] 6-nitro-2,3-diphenylquinoxaline (the product in No. 3): m.p.193-194°C; 1 HNMR (CDCl 3 , 300MHz) δ (ppm): 9.2 (d, 1H), 8.53 (dd, 1H), 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com