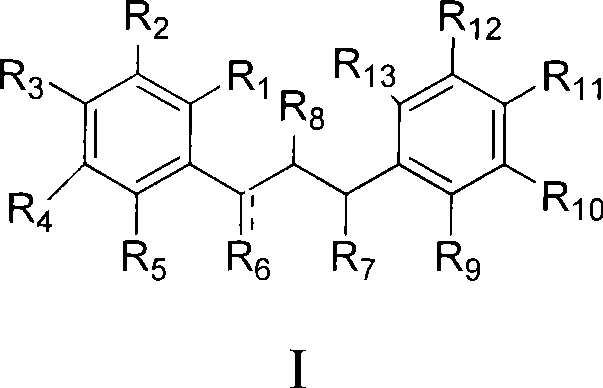
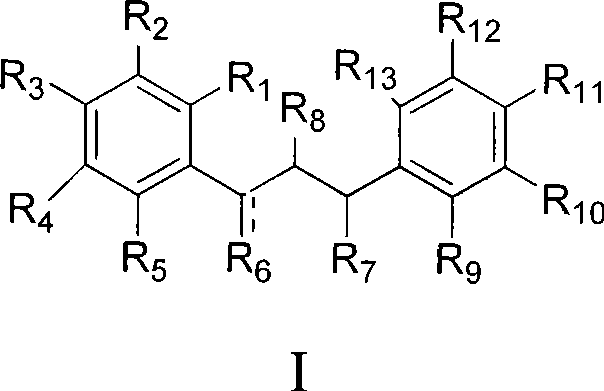
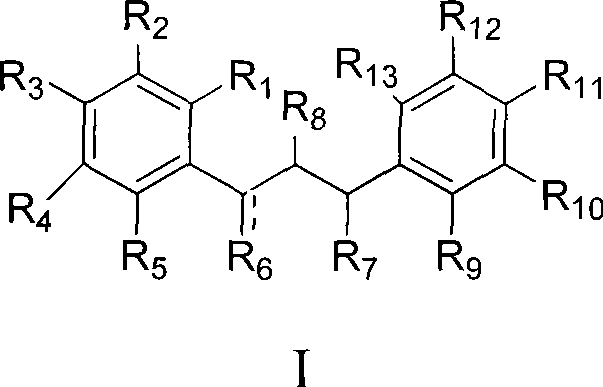
Dihydrochalcone derivates and use thereof
A technology of dihydrochalcones and derivatives, which is applied in the field of medicine and can solve problems that have not been seen before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of 3-(2-(benzyloxy)phenyl)-2-hydroxyl-1-(4-methoxyphenyl)-3-oxo-propanol acetate (DHC1)
[0048]
[0049] Using anisaldehyde and o-hydroxyacetophenone as starting materials, 2'-benzyloxy-4-methoxy Chalcones, then prepare DHC1 as follows:
[0050] 1) Preparation of 4-methoxy-2'-benzyloxychalcone epoxide
[0051] Take 3.4g of 4-methoxy-2'-benzyloxychalcone, add 50ml of acetone, 50ml of methanol, mix well and add 10ml of 2N NaOH at room temperature, 15ml of H 2 o 2 , stirred and reacted for 24 hours, then added 300ml of water to the reaction liquid, filtered, and the solid was recrystallized with acetone to obtain 3.2 g of white solid, with a yield of 89%.
[0052] 2) Preparation of 3-(2-(benzyloxy)phenyl)-2-hydroxyl-1-(4-methoxyphenyl)-3-oxo-propanol acetate (DHC1)
[0053] Take 500 mg of 4-methoxy-2'-benzyloxychalcone epoxide, add it into 10 ml of acetic acid, and stir the reaction at room temperature for 1 hour. Then, 100ml of water was ad...
Embodiment 2
[0056] Example 2: 3-(2-(Benzyloxy)phenyl)-2-hydroxy-1-(3,4-methylenedioxyphenyl)-3-oxo-propanol acetate (DHC2) preparation of
[0057]
[0058] Except that taking piperonal and o-hydroxyacetophenone as raw materials, all the other are the same as in Example 1. White crystals, melting point 157-161°C, yield 60%.
[0059] 1 HNMR (CDCl 3 )δ, ppm: 1.93 (3H, s, -CH 3 ); 4.01(1H, s, -OH); 5.18~5.28(2H, q, -CH2-); 5.36(1H, s, CH); 5.92(2H, s, -CH 2 -); 6.05~6.06(1H, d, CH); 6.48~6.52(1H, m, Ar-H); 6.59~6.61(1H, d.Ar-H); 6.68~6.69(1H, d.Ar- H); 7.10~7.14(2H, m, Ar-H); 7.39~7.47(6H, m, Ar-H); 7.54~7.60(1H, m, Ar-H), 7.80~7.83(1H, q, Ar-H)
[0060] MS(ESI)M+Na + =457.43
Embodiment 3
[0061] Example 3: Preparation of 1-(2-(benzyloxy)phenyl)-2,3-dihydroxy-3-(4-methoxyphenyl)acetone (DHC3)
[0062]
[0063] The preparation method of 4-methoxy-2'-benzyloxychalcone and 4-methoxyl-2'-benzyloxychalcone epoxide is the same as that of Example 1.
[0064] Take 100 mg of 4-methoxy-2'-benzyloxychalcone epoxide, add it to 10 ml of 15% NaOH solution, stir and react at room temperature for 3 hours, then pour the reaction solution into 50 ml of water, precipitate the solid and filter, Recrystallize from isopropanol to obtain a white solid with a melting point of 136-139°C.
[0065] 1 HNMR (CDCl 3 )δ, ppm: 3.77 (3H, s, -OCH 3 ); 3.78 (1H, d, -OH); 4.64 (1H, d, CH); 5.20~5.22 (2H, m, -OCH 2 -); 5.25(1H, s, CH); 6.75~6.78(2H, dd, Ar-H); 7.03~7.06(2H, dd, Ar-H); 7.09~7.14(2H, m, Ar-H) ;7.27~7.48(6H, m, Ar-H); 7.79~7.82(1H, m, Ar-H).
[0066] MS(ESI)M + =379.23
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com