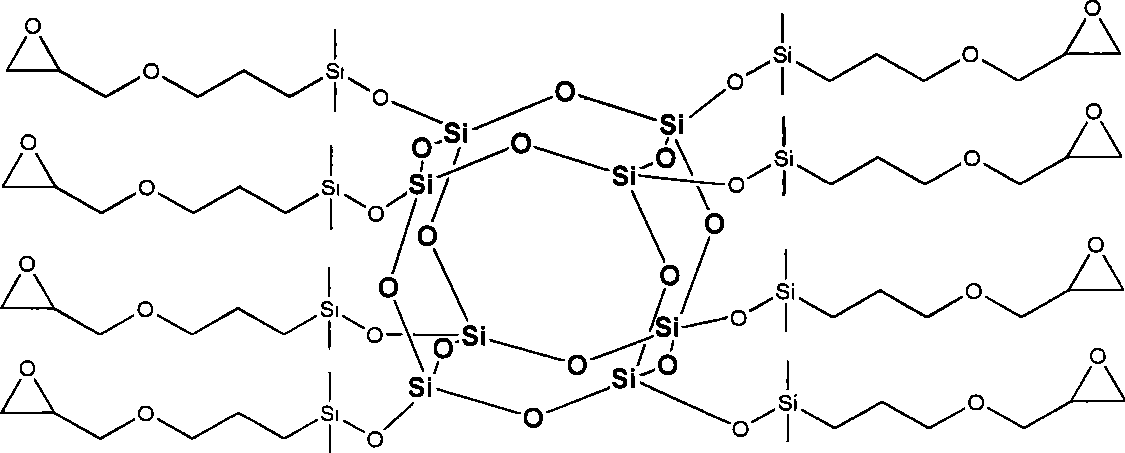
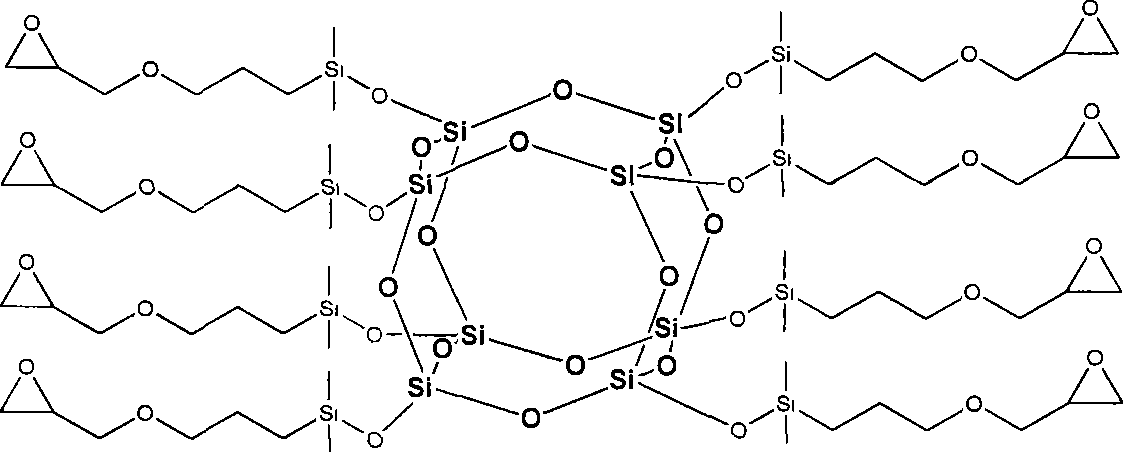
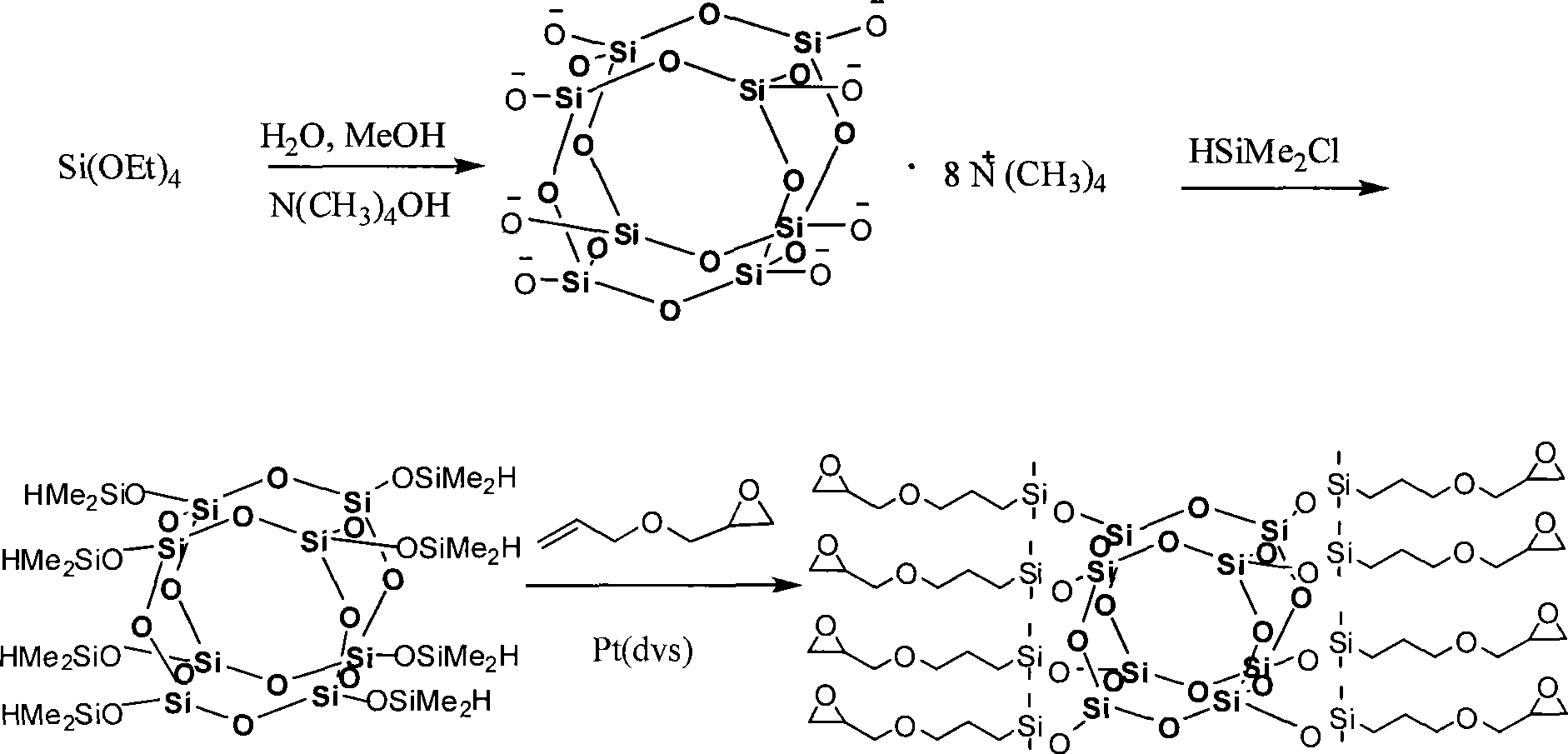
Octa-epoxy cage type sesquialter siloxane and preparation thereof
A technology of silsesquioxane and tetraethoxysilane, which is applied in the field of octaepoxy cage silsesquioxane and its preparation, can solve the problems affecting the dispersion and preparation of epoxy cage silsesquioxane The method has not been reported, and the problems such as affecting the use performance have achieved the effect of simple post-treatment method, improved compatibility, and improved reactivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] (1) Synthesis of hydrated octapolyclathosilicate (Tetramethylammonium silicate "Octanion').
[0024] At room temperature, 104.0 g of tetraethoxysilane was added dropwise to 590 g of 10 wt % tetramethylammonium hydroxide aqueous solution, stirred evenly, and then heated to 50° C., and reacted at this temperature for 15 hours to obtain a reaction liquid. Concentrate the reaction solution 10 times with a rotary evaporator, freeze in a refrigerator to crystallize, wash the crystals with acetone, and dry in vacuo to obtain 106.1 g of white hydrated octameric clathrosilicate, with a yield of 95%. Its nuclear magnetic resonance and infrared absorption analysis characterization data are: 29 Si-NMR (119MHz, CD 3 OD, 300K, ppm)-99.1; 13 C-NMR (159MHz, CD 3 OD, 300K, ppm) 15.10; 1 H-NMR (600MHz, CD 3 OD, 300K, ppm) 3.19; FTIR (cm -1 , KBr) 3393, 1643 (v N-O ), 1489 (v as C-N ), 1116 (v Si-O-Si ).
[0025] (2) Add 20ml of dimethylchlorosilane to a mixed solvent containing...
example 2
[0029] (1) Synthesis of hydrated octapolyclathosilicate (Tetramethylammonium silicate "Octanion').
[0030] At room temperature, 104.0 g of tetraethoxysilane was added dropwise to 936 g of 10 wt % tetramethylammonium hydroxide aqueous solution, stirred evenly, then heated to 60° C., and continued to react for 10 h to obtain a reaction liquid. Concentrate the reaction solution to 8 times with a rotary evaporator, freeze in a refrigerator to crystallize, wash the crystals with acetone, and dry them in vacuum to obtain 108.3 g of white hydrated octameric clathrosilicate, with a yield of 97%. Its nuclear magnetic resonance and infrared absorption analysis characterization data are: 29 Si-NMR (119MHz, CD 3 OD, 300K, ppm)-99.1; 13 C-NMR (159MHz, CD 3 OD, 300K, ppm) 15.10; 1 H-NMR (600MHz, CD 3 OD, 300K, ppm) 3.19; FTIR (cm -1 , KBr) 3393, 1643 (v N-O ), 1489 (v asC-N ), 1116 (v Si-O-Si ).
[0031] (2) Add 30ml of dimethylchlorosilane to a mixed solvent containing 90ml of n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com