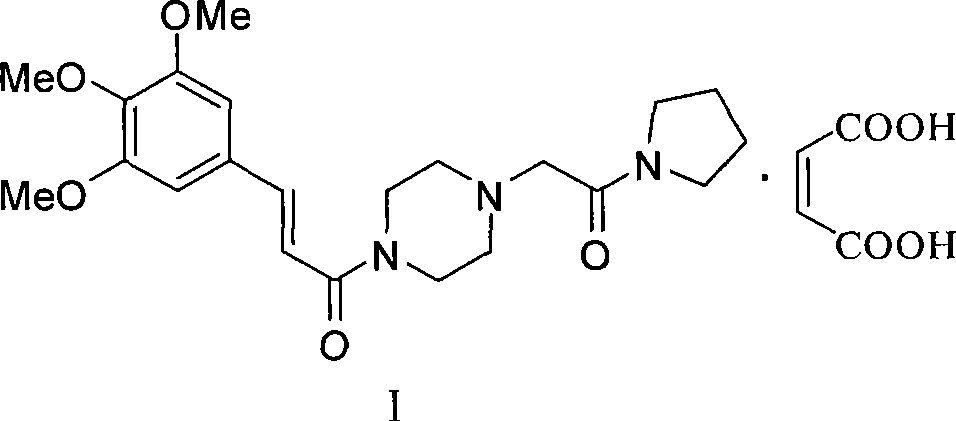
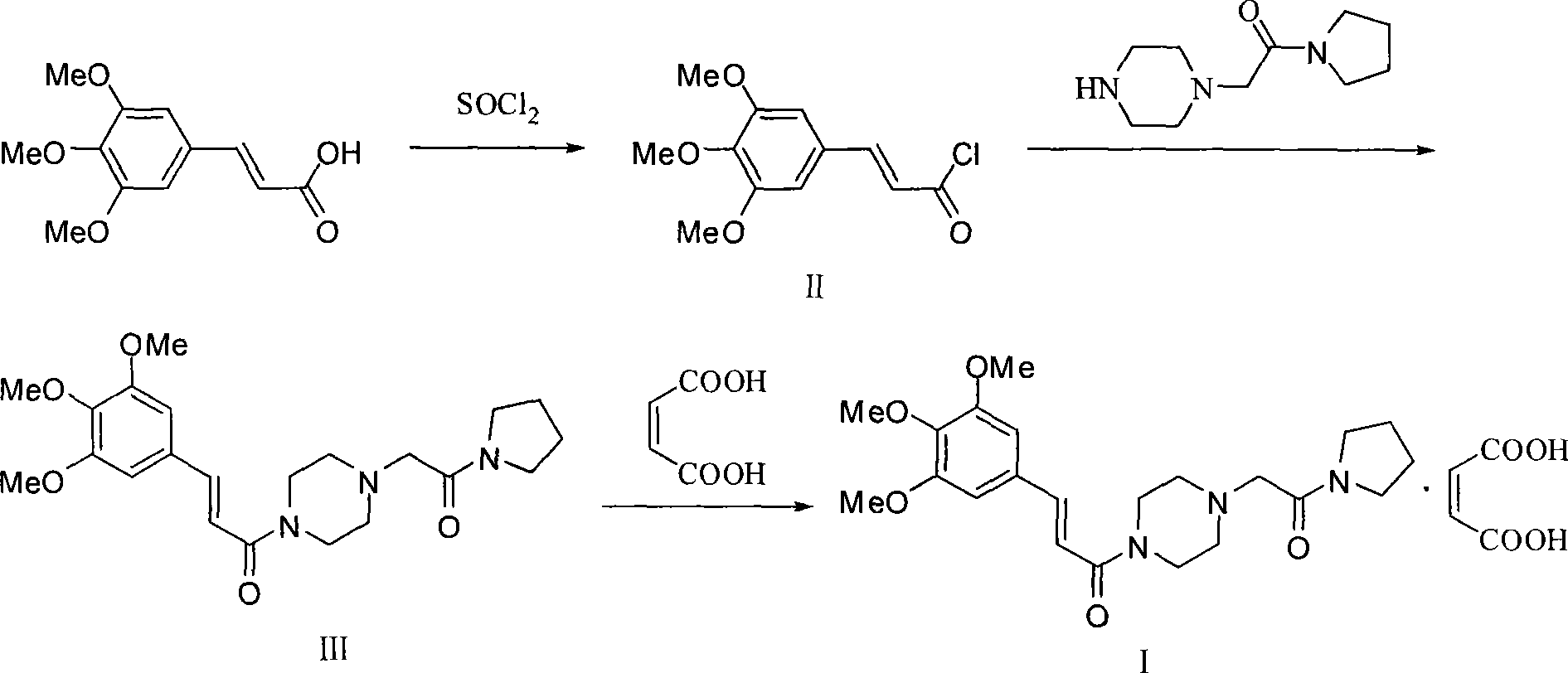
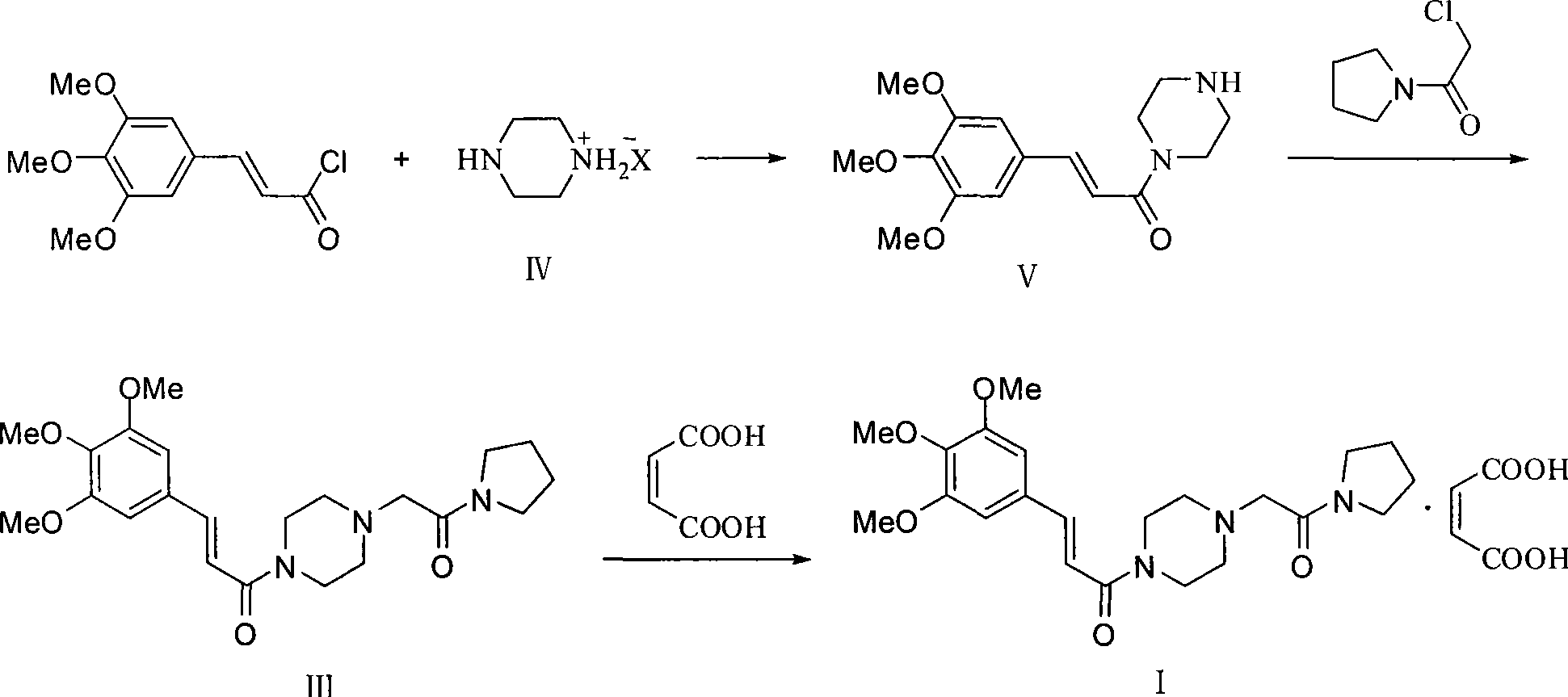
Synthesis of cinepazide maleate
A technology of cinepazide maleate and a synthesis method, applied in the field of medicine, can solve problems such as long steps and low yield, and achieve the effects of mild reaction conditions, shortening reaction steps, and reducing equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Preparation of cinepazide free base
[0038] Reaction formula:
[0039]
[0040] operating:
[0041] 1.20g (0.005mol) of trans 3,4,5-trimethoxycinnamic acid, 1.01g (2mol.eq.) of triethylamine, and 20ml of dichloromethane were stirred and dissolved at -10°C, and 0.65g ( 1.2mol.eq.) 5ml dichloromethane solution of ethyl chloroformate, keep stirring and react for 1 hour (TLC follows the reaction process, methanol is the developing solvent), then slowly drop 0.99g (1.0mol.eq.) 1-[ A solution of (1-tetrahydropyrrolecarbonyl)methyl]piperazine in 10 ml of dichloromethane was incubated and stirred for 3 hours (TLC followed the progress of the reaction, methanol was the developing solvent). After the reaction is complete, the reaction solution is acidified with 10% HCl to PH=1~2, and the organic layer is separated; the acid layer is washed with dichloromethane 10ml*2 times and then basified with 20% sodium hydroxide to PH=12~14, and then Extract 20ml*4 times with dichloromethan...
Embodiment 2
[0053] Reaction formula:
[0054]
[0055] operating:
[0056] 1.20g (0.005mol) of trans 3,4,5-trimethoxycinnamic acid, 1.01g (2mol.eq.) of triethylamine, and 20ml of dichloromethane were stirred and dissolved at 20°C, and 0.65g (1.2 mol.eq.) 5ml dichloromethane solution of ethyl chloroformate, keep stirring and react for 1 hour (TLC follows the reaction process, methanol is the developing solvent), then slowly drop 0.99g(1.0mol.eq.)1-[( A solution of 1-tetrahydropyrrolecarbonyl)methyl]piperazine in 10 ml of dichloromethane was incubated and stirred for 3 hours (TLC followed the progress of the reaction, methanol was the developing solvent). After the reaction is complete, the reaction solution is acidified with 10% HCl to PH=1~2, and the organic layer is separated; the acid layer is washed with dichloromethane 10ml*2 times and then basified with 20% sodium hydroxide to PH=12~14, and then Extract 20ml*4 times with dichloromethane, and then wash with saturated sodium bicarbonate ...
Embodiment 3
[0061] Reaction formula:
[0062]
[0063] operating:
[0064]1.20g (0.005mol) of trans 3,4,5-trimethoxycinnamic acid, 1.01g (2mol.eq.) of triethylamine, 20ml of dichloromethane were stirred and dissolved at -10°C, and 0.57g ( 1.2mol.eq.) 5ml dichloromethane solution of methyl chloroformate, keep warm and stir and react for 1 hour (TLC follows the reaction process, methanol is the developing solvent), then slowly drop 0.99g (1.0mol.eq.) 1-[ A solution of (1-tetrahydropyrrolecarbonyl)methyl]piperazine in 10 ml of dichloromethane was incubated and stirred for 3 hours (TLC followed the reaction process, methanol was the developing solvent). After the reaction is completed, the reaction solution is acidified with 10% HCl to PH=1~2, and the organic layer is separated; the acid layer is washed with dichloromethane 10ml*2 times and then basified with 20% sodium hydroxide to PH=12~14, and then Extract 20ml*4 times with dichloromethane, then wash 30ml*2 times with saturated sodium bicarb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com