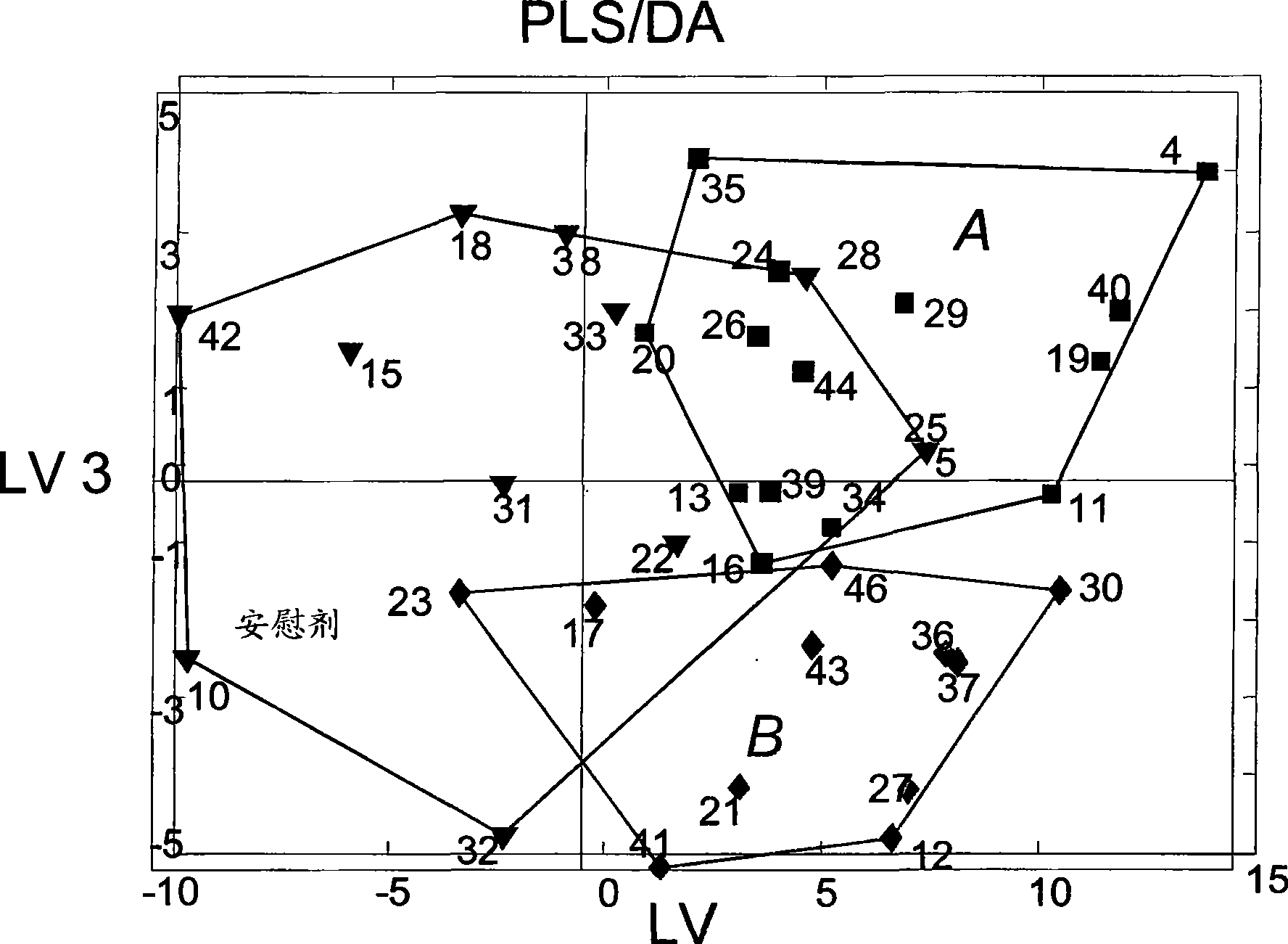
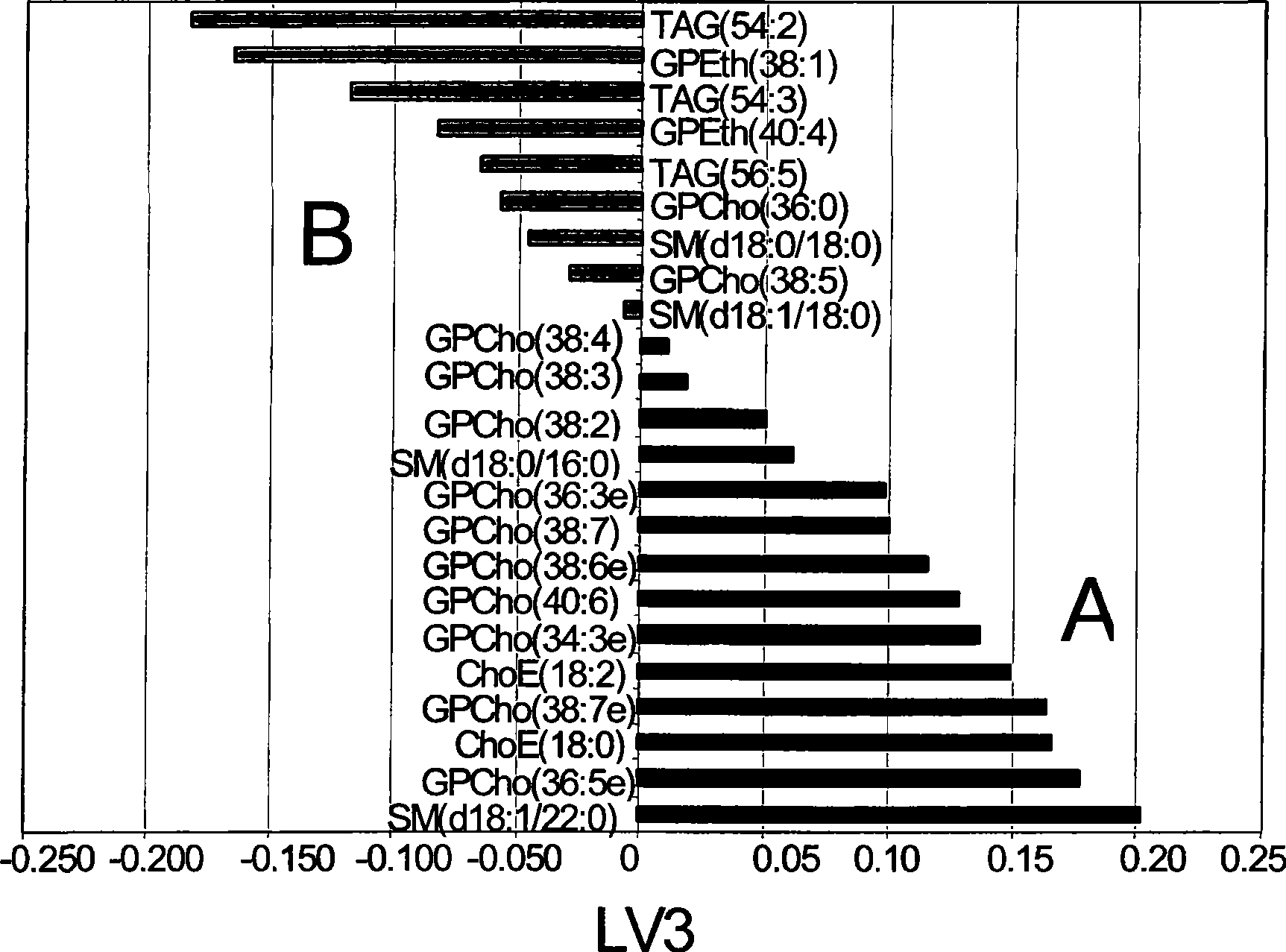
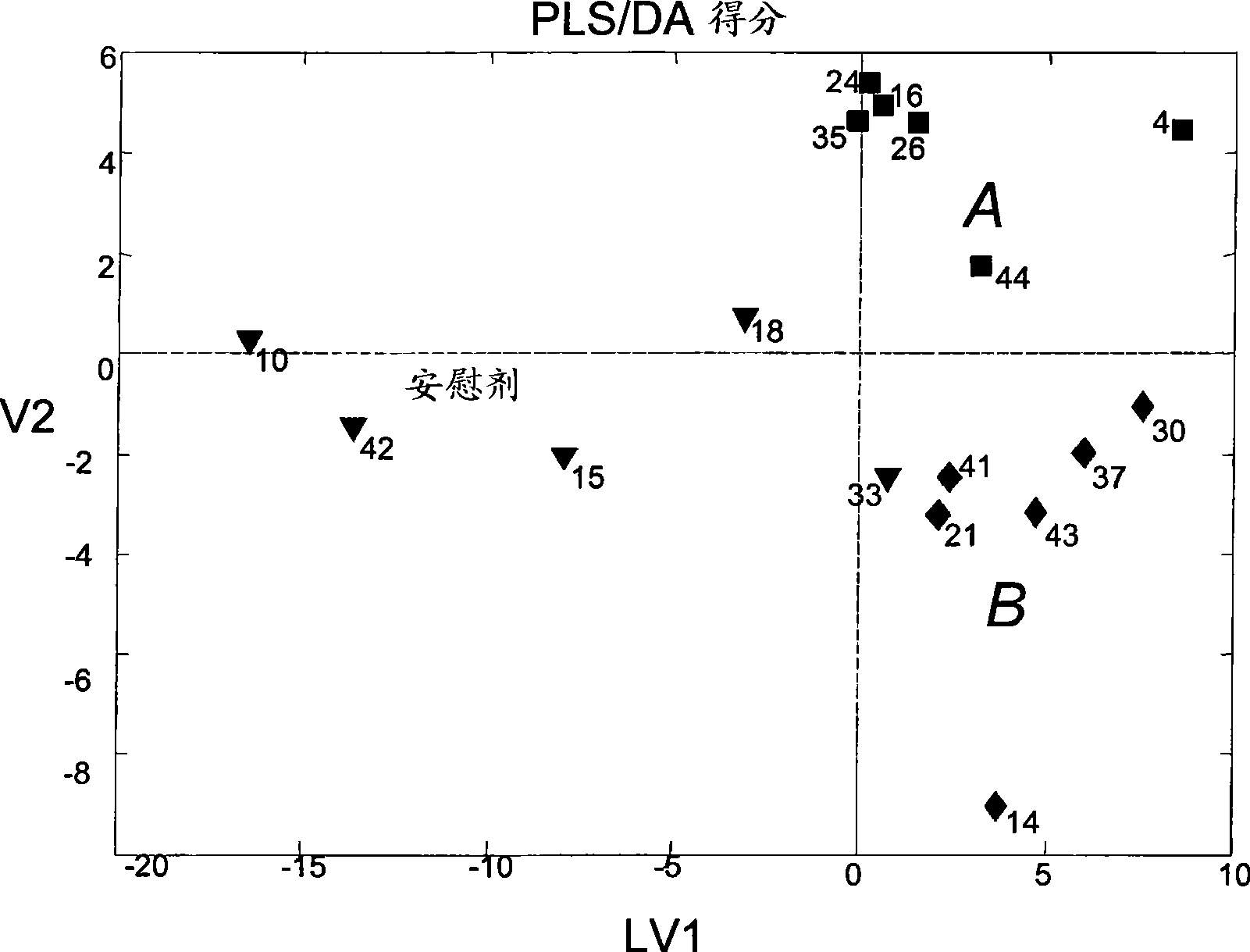
Diagnostic method for myopathy
A myopathy and lipid technology, which is applied in the field of diagnosis of myopathy induced by statins, can solve the problems that there are no diagnostic methods or clinical tests for diagnosing non-symptomatic myopathy, and the risk of myopathy cannot be estimated in patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Gene expression analysis
[0054] gene expression
[0055] According to the instructions given, use Human-6 Expression Bead Chip Analysis Microarray experiments were performed on 46,000 known genes, candidate genes and splice variants (Illumina, San Diego, CA, USA). Biopsy samples were homogenized using an Ultra-Turrax (IKA Turrax T8 / S8N-5G, IKA-Werke, Staufen, Germany). Total RNA was extracted using TRIzol (#15596-018, Invitrogen Corporation, Carlsbad, CA). DNase treatment and secondary RNA purification were performed using Qiagen kits (#74106 and #79254, Qiagen GmbH, Hilden, Germany) according to the given instructions.
[0056] A 200 ng aliquot of total RNA from each sample was amplified as cDNA using Ambion's Illumina RNA Amplification Kit (Cat. No. 11755, Ambion, Inc., Austin, TX, USA) according to the instructions. Perform an overnight (14h) in vitro transcription (IVT) reaction from cDNA to cRNA containing biotin-11-dUTP (PerkinElmer, Cat. No. PC ...
Embodiment 2
[0060] Example 2.RT-PCR analysis
[0061] Based on hierarchical cluster analysis (as described in Example 1), 20 genes were selected for further RT-PCR controls to identify a gene expression-based fingerprint of the effect of statins on human skeletal muscle.
[0062] Microarray expression results recorded in the simvastatin group were validated by real-time quantitative TaqMan PCR (n=5, one case did not have enough muscle RNA for PCR). Use previously purified cRNA as a starting material for cDNA synthesis. 1000 ng-18 μl aliquots of cRNA were mixed with 1 μl Promega random primer (C1181, Promega U.S., Madison, WI, USA) and incubated at +70°C for 10 minutes. The following reagents were added to bring the total reaction volume to 25 μl: 1 μl of 10 μM dNTP mix (F09892, Applied Biosystems, Foster City, CA, USA), 1 μl of Promega M-MLV reverse transcriptase 200 U / μl (M3682) and 4 μl of M-MLV Reverse Transcription (RT) 5X Reaction Buffer. Finally, incubation was performed in the f...
Embodiment 3
[0065] Example 3. Lipidomic analysis of plasma
[0066] Aliquots of internal standard mixture (10ml) containing 11 lipid classes and 0.05M NaCl (10ml) were added to plasma samples (10ml) and lipids were extracted with chloroform / methanol (2:1, 100ml) . After vortexing (2 minutes), standing (1 hour) and centrifugation (10,000 RPM, 3 minutes), the lower layer was separated and a standard mixture (10 ml) containing 3 labeled standard lipids was added to the extract (inner Standards and external standards are listed in the appendix). The order of samples for LC / MS analysis was determined randomly.
[0067] Using Waters Q-Tof Premier mass spectrometer combined with Acquity ultra-high performance liquid chromatography (LC) TM (UPLC) to analyze lipid extracts. The column was maintained at 50°C and was an Acquity UPLC™ BEH C18 10 x 50mm containing 1.7mm particles. Binary solvent system (binary solvent system) includes: A. Water (1% 1M NH 4 Ac, 0.1% HCOOH); B. LC / MS grade (Rathbu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com