Polysaccharide-liposome and preparation method and purpose thereof
A liposome and polysaccharide technology, which is applied in the preparation of polysaccharide liposomes and the field of lentinan liposomes, can solve difficult problems and achieve obvious targeting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of Liposome 1:
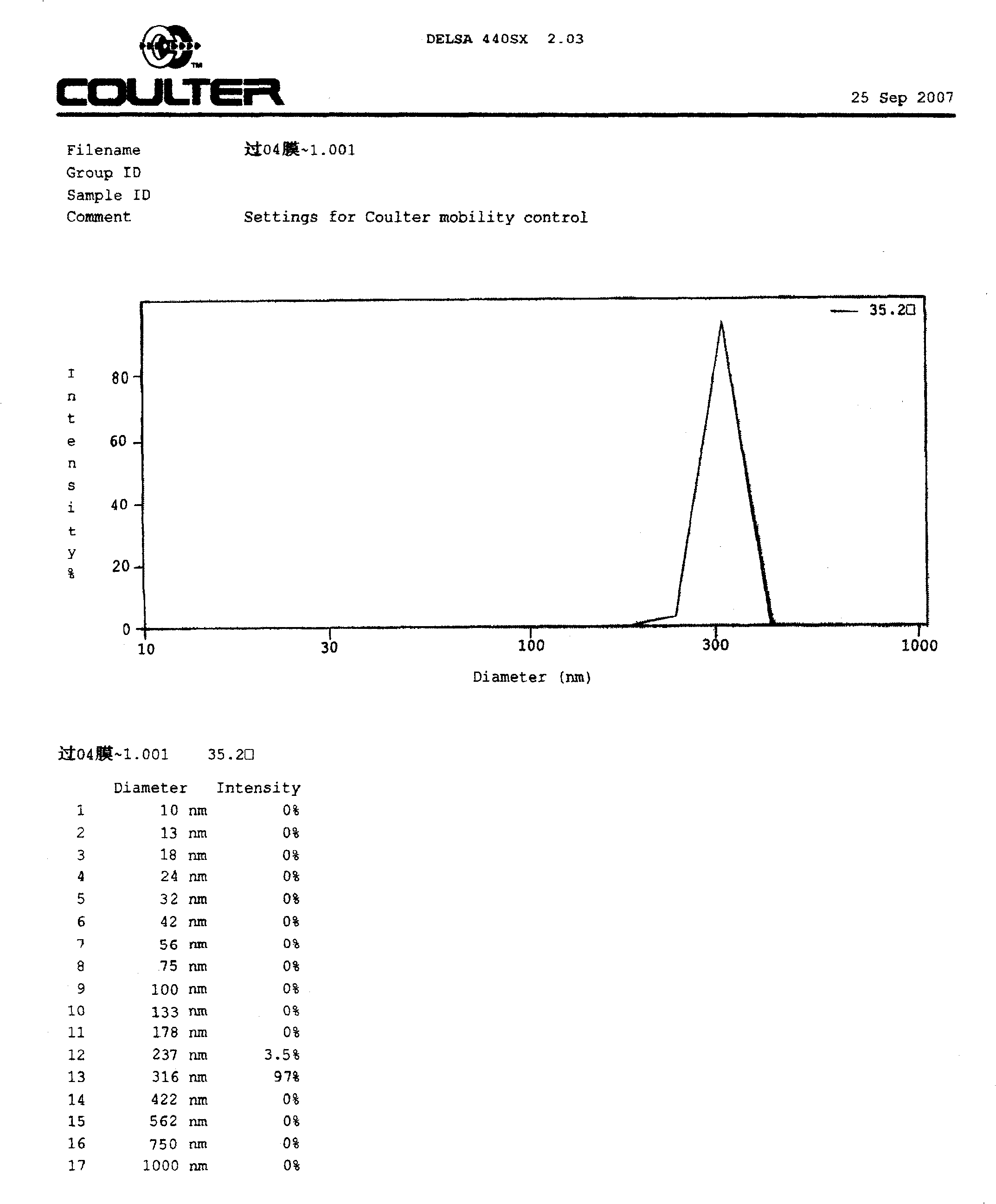
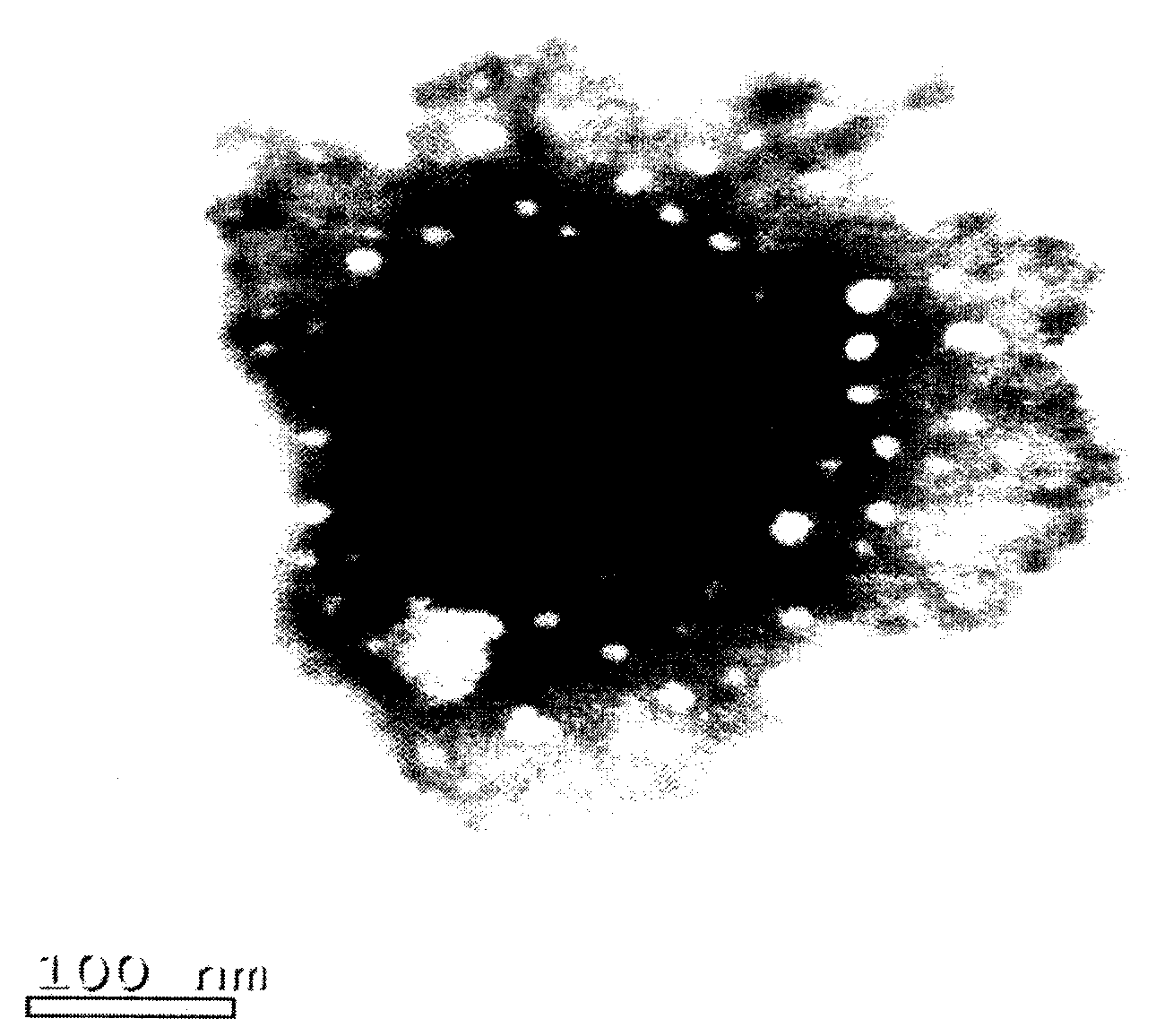
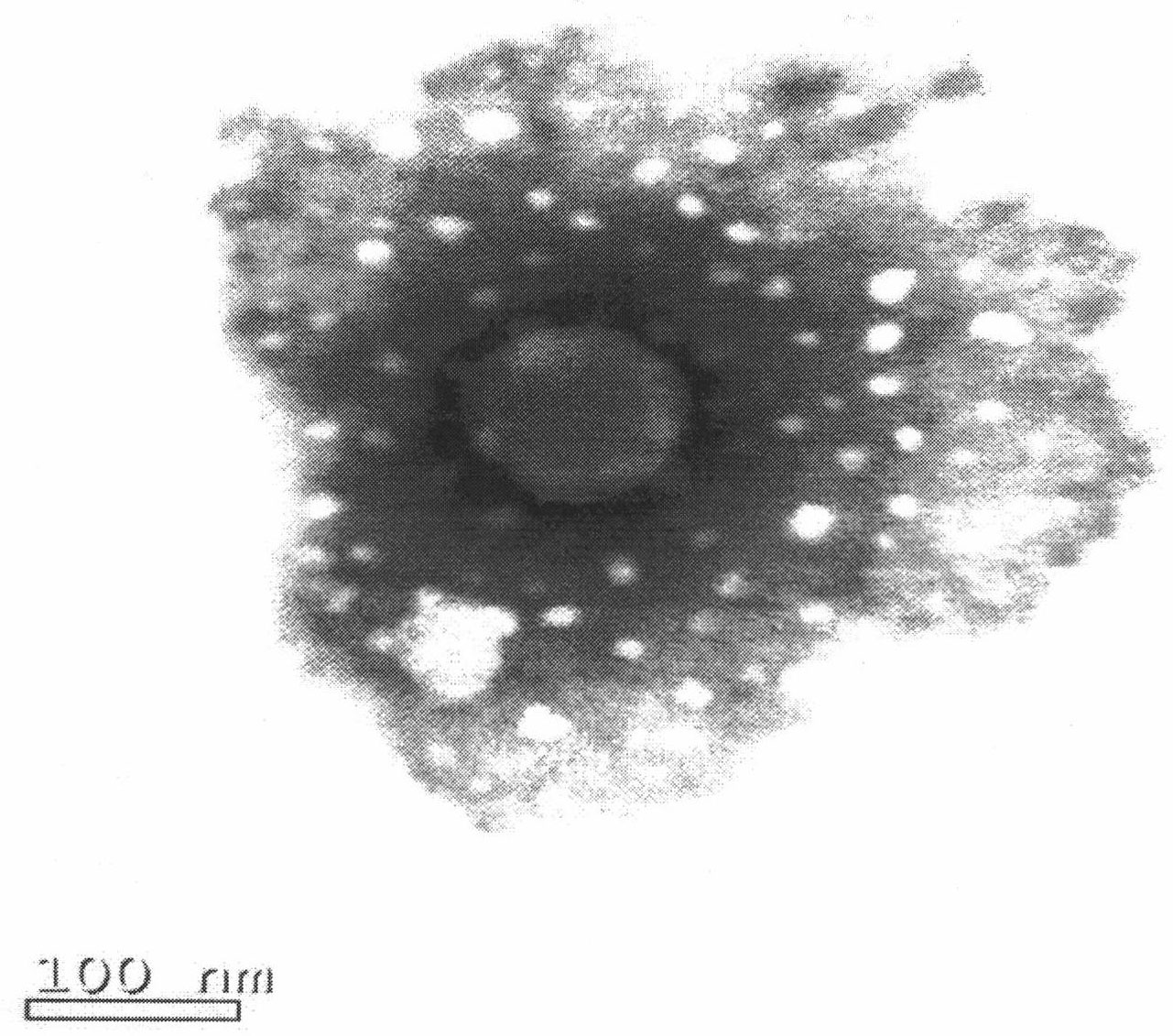
[0063] Weigh 8.0 g of soybean lecithin, 1.0 g of cholesterol, 0.2 g of polyethylene glycol 2000 and 0.2 g of vitamin E, add 900 ml of chloroform, stir and dissolve to obtain an oil phase mixture. Weigh 0.1g injection-grade lentinan and 0.9g sodium deoxycholate, add 300ml of water for injection, and add an appropriate amount of 0.1mol / L sodium hydroxide solution until the lentinan dissolves, and adjust the pH of the solution to 7.0 with 0.1mol / L hydrochloric acid ~8.0, an aqueous phase mixture was obtained. Under cooling in an ice-water bath, slowly add the water phase mixture into the oil phase mixture, and then ultrasonically emulsify intermittently for about 5 minutes to obtain a milky white uniform W / O emulsion. The solvent was removed by rotary evaporation under reduced pressure at 40°C under the protection of nitrogen to obtain a pale yellow thick jelly, which was added with 200ml of 0.9% sodium chloride solution and incubated a...
Embodiment 2
[0074] Preparation: Weigh 8.0 g of soybean lecithin, 1.0 g of cholesterol, 0.2 g of polyethylene glycol 2000 and 0.2 g of vitamin E, add 900 ml of chloroform, stir to dissolve, and obtain an oil phase mixture. Weigh 0.1g of injection-grade lentinan and 0.9g of sodium deoxycholate, add 300ml of water for injection, and add an appropriate amount of 0.1mol / L sodium hydroxide solution until the lentinan dissolves, and adjust the pH value to 7.0~ with 0.1mol / L hydrochloric acid 8.0, an aqueous phase mixture was obtained. Under cooling in an ice-water bath, slowly add the water phase mixture into the oil phase mixture, and then ultrasonically emulsify intermittently for about 5 minutes to obtain a milky white uniform W / O emulsion. Remove the solvent by rotary evaporation under reduced pressure at 40°C under the protection of nitrogen to obtain a light yellow thick jelly, add 200ml of 2% mannitol in phosphate buffer (pH 6.0-6.5), and incubate at 40°C for 1h to obtain liposome ...
Embodiment 3
[0080] Preparation: Weigh 6.1g of soybean lecithin, 0.8g of cholesterol, 0.2g of polyethylene glycol 1000 and 0.2g of vitamin E, add 900ml of chloroform, stir to dissolve, and obtain an oil phase mixture. Weigh 0.1g of injection-grade lentinan and 0.5g of sodium deoxycholate, add 300ml of water for injection, and add an appropriate amount of 0.1mol / L sodium hydroxide solution until the lentinan dissolves, and adjust the pH value to 7.0~ with 0.1mol / L hydrochloric acid 8.0, an aqueous phase mixture was obtained. Under cooling in an ice-water bath, slowly add the water phase mixture into the oil phase mixture, and then ultrasonically emulsify intermittently for about 6 minutes to obtain a milky white uniform W / O emulsion. The solvent was removed by rotary evaporation under reduced pressure at 35°C under the protection of nitrogen to obtain a light yellow thick jelly. Add 200ml of phosphate buffer solution with a pH value of 6.0 to 6.5, and incubate at 40°C for 1.5h to obtain a l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com