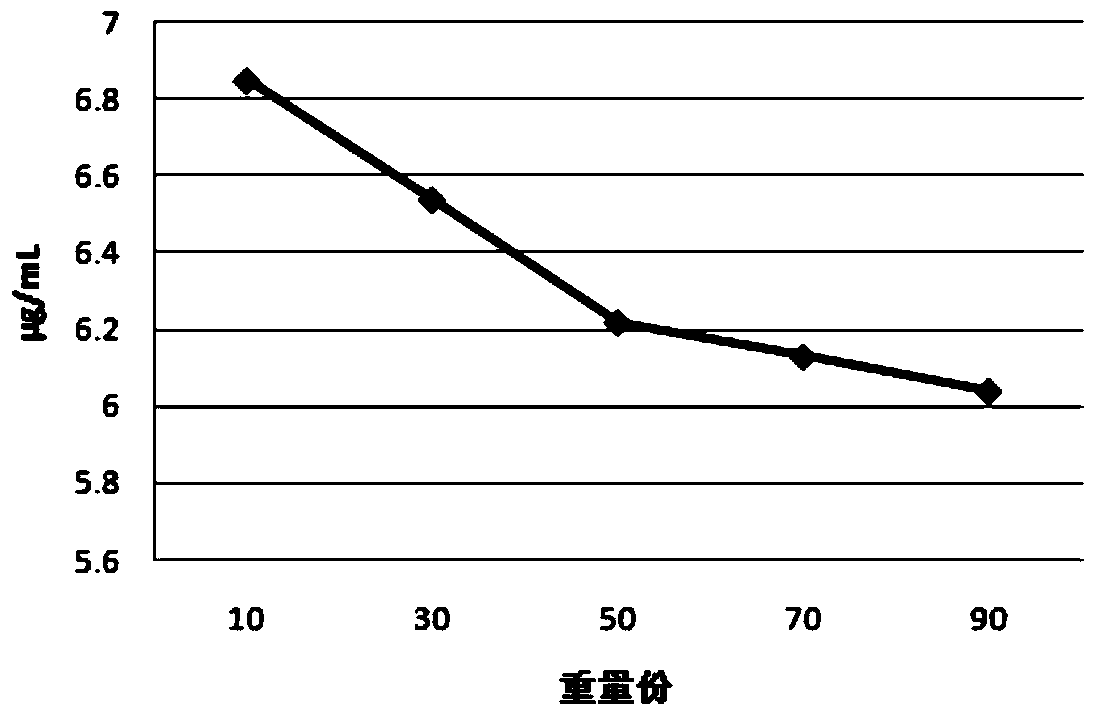
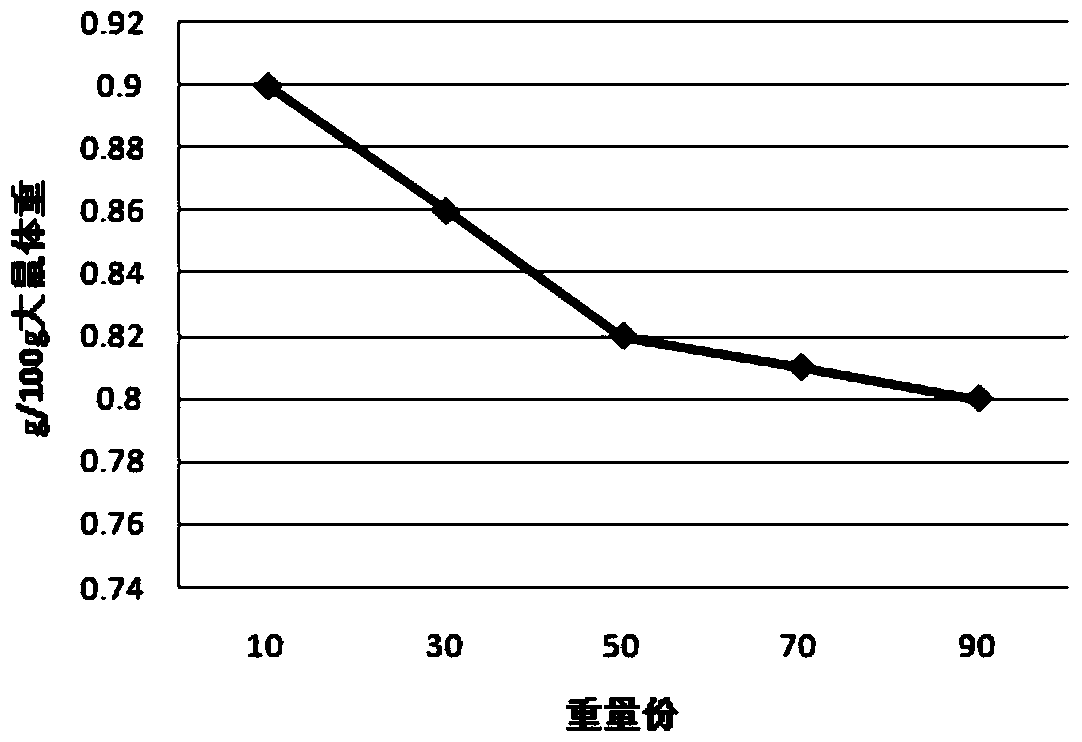
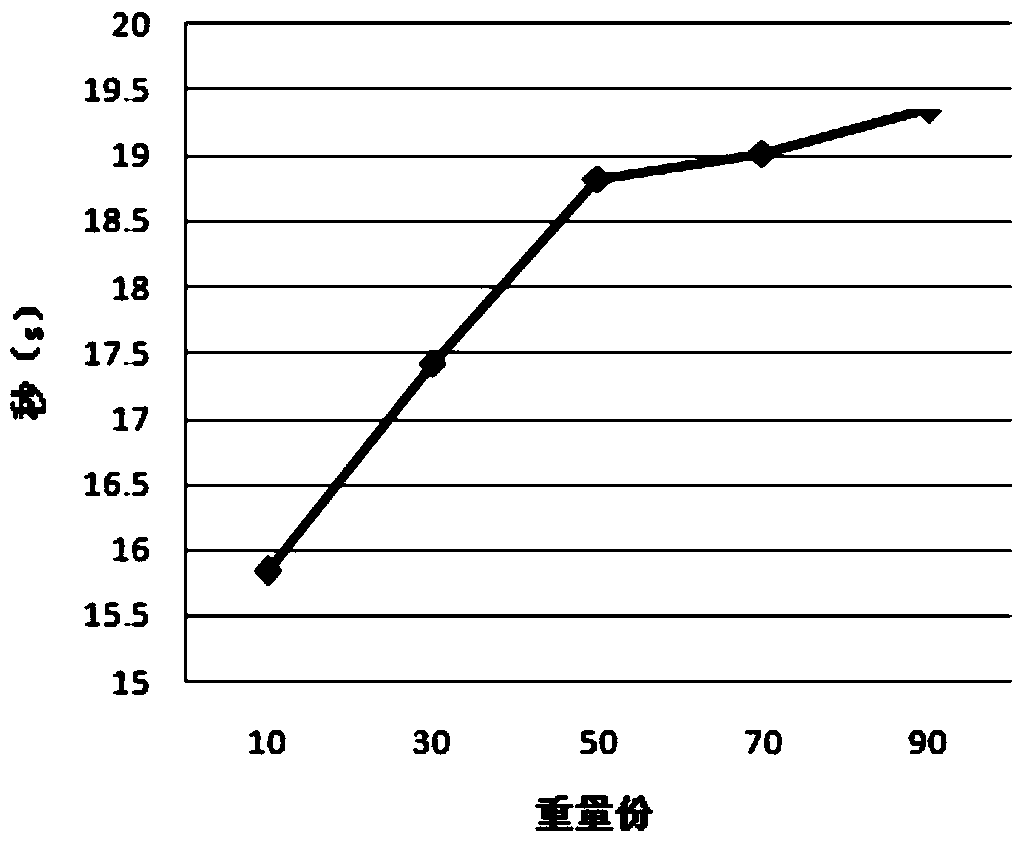
A Tibetan medicine composition for treating white pulse disease and its preparation method
A composition and white pulse disease technology, which is applied in the direction of drug combination, pharmaceutical formula, and medical raw materials derived from crustaceans, can solve the problems of long disintegration time of products, cumbersome production process, and unsuitability for modern production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0059] Experimental example 1, alcohol extraction orthogonal test
[0060] In ethanol extraction, the main factors affecting the extraction effect are extraction time, extraction times, alcohol addition and ethanol concentration. Three levels are selected for each factor, and an orthogonal experiment of four factors and three levels is carried out to screen the optimal alcohol extraction process. See Table 1 for the design.
[0061] Table 1 Factor level table
[0062]
[0063]
[0064] Test method and results: Accurately weigh 3.75g of longan longan, 10g of sandalwood, 5g of black cumin, 41.25g of balsamic, 10g of galangal, 10g of woody, 10g of Tibetan woody, 12.5g of agarwood, 5g of clove, and 5g of nutmeg , cardamom 5g, grass fruit 3.75g, parsley 5g, cinnamon 6.25g, a total of 132.5g, after extracting the volatile oil, add 12.5g of safflower, 7.5g of cassia seed, 18.75g of short ear grass, 6.25g of ambrette seed, total 177.5g, with L 9 (3 4 ) Orthogonal table arran...
experiment example 2
[0073] Experimental example 2, water extraction orthogonal test
[0074] In water extraction, the factors that affect the extraction effect mainly include extraction time, extraction frequency and water addition. For each factor, three levels are selected, and an orthogonal test of three factors and three levels is carried out to screen the optimal water extraction process. See Table 4 for the design.
[0075] Table 4 Factor level table
[0076]
[0077] Accurately weigh 16.25g of myrobalan, 16.25g of emblica, and 12.5g of myrobalan (pitted), 45g in total, and use L 9 (3 4 ) Orthogonal table arrangement test for reflux extraction, filter the extract, filter, combine the extracts, measure the total volume, take 50ml, measure the yield of dry cream, take the extract, and measure the total polyphenol content.
[0078] For the screening index, the main evaluation index of the extraction process is the total polyphenol content, and the weight coefficient is set to 0.7, and th...
experiment example 3
[0086] Experimental example 3, volatile oil inclusion orthogonal test
[0087] In order to retain the volatile oil components as much as possible and ensure the efficacy, β-cyclodextrin was used to clathrate the volatile oil according to the preliminary test results. The ratio of β-CD to oil during inclusion (A), inclusion temperature (B), inclusion time (C) and β-CD:water (D) were investigated, and the factor levels are shown in Table 7.
[0088] Table 7 Factor level table
[0089]
[0090] Follow L9 (3 4 ) Orthogonal experiment table Arrange experiments, accurately weigh β-cyclodextrin, put it into a stoppered Erlenmeyer flask, add distilled water, heat in a boiling water bath to dissolve, lower to 40°C, and stir on a magnetic stirrer for 30 minutes. Accurately measure 2ml of volatile oil, dilute it with ethanol at a ratio of 1:1, drop the diluted volatile oil into the β-cyclodextrin solution with a dropper, stopper, and stir at constant temperature until the specified ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com